Comparative genomics
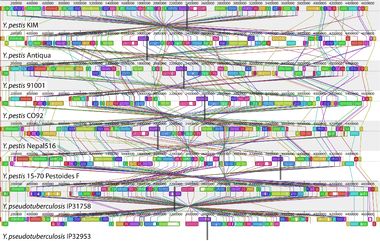
Comparative genomics is a field of biological research in which the genomic features of different organisms are compared.[2][3] The genomic features may include the DNA sequence, genes, gene order, regulatory sequences, and other genomic structural landmarks.[3] In this branch of genomics, whole or large parts of genomes resulting from genome projects are compared to study basic biological similarities and differences as well as evolutionary relationships between organisms.[2][4][5] The major principle of comparative genomics is that common features of two organisms will often be encoded within the DNA that is evolutionarily conserved between them.[6] Therefore, comparative genomic approaches start with making some form of alignment of genome sequences and looking for orthologous sequences (sequences that share a common ancestry) in the aligned genomes and checking to what extent those sequences are conserved. Based on these, genome and molecular evolution are inferred and this may in turn be put in the context of, for example, phenotypic evolution or population genetics.[7]
Virtually started as soon as the whole genomes of two organisms became available (that is, the genomes of the bacteria Haemophilus influenzae and Mycoplasma genitalium) in 1995, comparative genomics is now a standard component of the analysis of every new genome sequence.[2][8] With the explosion in the number of genome project due to the advancements in DNA sequencing technologies, particularly the next-generation sequencing methods in late 2000s, this field has become more sophisticated, making it possible to deal with many genomes in a single study.[9] Comparative genomics has revealed high levels of similarity between closely related organisms, such as humans and chimpanzees, and, more surprisingly, similarity between seemingly distantly related organisms, such as humans and the yeast Saccharomyces cerevisiae.[4] It has also showed the extreme diversity of the gene composition in different evolutionary lineages.[8]
History
See also: History of genomics
Comparative genomics has a root in the comparison of virus genomes in the early 1980s.[8] For example, small RNA viruses infecting animals (picornaviruses) and those infecting plants (cowpea mosaic virus) were compared and turned out to share significant sequence similarity and, in part, the order of their genes.[10] In 1986, the first comparative genomic study at a larger scale was published, comparing the genomes of varicella-zoster virus and Epstein-Barr virus that contained more than 100 genes each.[11]
The first complete genome sequence of a cellular organism, that of Haemophilus influenzae Rd, was published in 1995.[12] The second genome sequencing paper was of the small parasitic bacterium Mycoplasma genitalium published in the same year.[13] Starting from this paper, reports on new genomes inevitably became comparative-genomic studies.[8]
The first high-resolution whole genome comparison system was developed in 1998 by Art Delcher, Simon Kasif and Steven Salzberg and applied to the comparison of entire highly related microbial organisms with their collaborators at the Institute for Genomic Research (TIGR). The system is called MUMMER and was described in a publication in Nucleic Acids Research in 1999. The system helps researchers to identify large rearrangements, single base mutations, reversals, tandem repeat expansions and other polymorphisms. In bacteria, MUMMER enables the identification of polymorphisms that are responsible for virulence, pathogenicity, and anti-biotic resistance. The system was also applied to the Minimal Organism Project at TIGR and subsequently to many other comparative genomics projects.
Saccharomyces cerevisiae, the baker's yeast, was the first eukaryote to have its complete genome sequence published in 1996.[14] After the publication of the roundworm Caenorhabditis elegans genome in 1998[15] and together with the fruit fly Drosophila melanogaster genome in 2000,[16] Gerald M. Rubin and his team published a paper titled "Comparative Genomics of the Eukaryotes", in which they compared the genomes of the eukaryotes D. melanogaster, C. elegans, and S. cerevisiae, as well as the prokaryote H. influenzae.[17] At the same time, Bonnie Berger, Eric Lander, and their team published a paper on whole-genome comparison of human and mouse.[18]
With the publication of the large genomes of vertebrates in the 2000s, including human, the Japanese pufferfish Takifugu rubripes, and mouse, precomputed results of large genome comparisons have been released for downloading or for visualization in a genome browser. Instead of undertaking their own analyses, most biologists can access these large cross-species comparisons and avoid the impracticality caused by the size of the genomes.[19]
Next-generation sequencing methods, which were first introduced in 2007, have produced an enormous amount of genomic data and have allowed researchers to generate multiple (prokaryotic) draft genome sequences at once. These methods can also quickly uncover single-nucleotide polymorphisms, insertions and deletions by mapping unassembled reads against a well annotated reference genome, and thus provide a list of possible gene differences that may be the basis for any functional variation among strains.[9]
Evolutionary principles
One character of biology is evolution, evolutionary theory is also the theoretical foundation of comparative genomics, and at the same time the results of comparative genomics unprecedentedly enriched and developed the theory of evolution. When two or more of the genome sequence are compared, you can get the evolutionary relationships of the sequences in a phylogenetic tree. Based on a variety of biological genome data and the study of vertical and horizontal evolution processes, one can understand vital parts of the gene structure and its regulatory function. But in a genome about 1.5% ~ 14.5% of the genes are related to the "lateral migration phenomenon", namely the transfer of a gene between populations which can exist at the same time. Thus, the differences in sequences have nothing to do with evolution.
Similarity of related genomes is the basis of comparative genomics. If two creatures have a recent common ancestor, the differences between the two species genomes are evolved from the ancestors’ genome. The closer the relationship between two organisms, the higher the similiarities between their genomes. If there is close relationship between them, then their genome will display a linear behaviour (synteny), namely some or all of the genetic sequences are conserved. Thus, the genome sequences can be used to identify gene function, by analyzing their homology (sequence similarity) to genes of known function.
Orthologous sequences are related sequences in different species: a gene exists in the original species, the species divided into two species, so genes in new species are orthologous to the sequence in the original species. Paralogous sequences are separated by gene cloning (gene duplication): if a particular gene in the genome is copied, then the copy of the two sequences is paralogous to the original gene. A pair of orthologous sequences is called orthologous pairs (orthologs), a pair of paralogous sequence is called collateral pairs (paralogs). Orthologous pairs usually have the same or similar function, which is not necessarily the case for collateral pairs. In collateral pairs, the sequences tend to evolve into having different functions.
Comparative genomics exploits both similarities and differences in the proteins, RNA, and regulatory regions of different organisms to infer how selection has acted upon these elements. Those elements that are responsible for similarities between different species should be conserved through time (stabilizing selection), while those elements responsible for differences among species should be divergent (positive selection). Finally, those elements that are unimportant to the evolutionary success of the organism will be unconserved (selection is neutral).
One of the important goals of the field is the identification of the mechanisms of eukaryotic genome evolution. It is however often complicated by the multiplicity of events that have taken place throughout the history of individual lineages, leaving only distorted and superimposed traces in the genome of each living organism. For this reason comparative genomics studies of small model organisms (for example the model Caenorhabditis elegans and closely related Caenorhabditis briggsae) are of great importance to advance our understanding of general mechanisms of evolution.[20][21]
Methods
Computational approaches to genome comparison have recently become a common research topic in computer science. A public collection of case studies and demonstrations is growing, ranging from whole genome comparisons to gene expression analysis.[22] This has increased the introduction of different ideas, including concepts from systems and control, information theory, strings analysis and data mining.[23] It is anticipated that computational approaches will become and remain a standard topic for research and teaching, while multiple courses will begin training students to be fluent in both topics.[24]
Tools
Computational tools for analyzing sequences and complete genomes are developed quickly due to the availability of large amount of genomic data. At the same time, comparative analysis tools are progressed and improved. In the challenges about these analyses, it is very important to visualize the comparative results.[25]
Visualization of sequence conservation is a tough task of comparative sequence analysis. As we know, it is highly inefficient to examine the alignment of long genomic regions manually. Internet-based genome browsers provide many useful tools for investigating genomic sequences due to integrating all sequence-based biological information on genomic regions. When we extract large amount of relevant biological data, they can be very easy to use and less time-consuming.[25]
- UCSC Browser: This site contains the reference sequence and working draft assemblies for a large collection of genomes.[26]
- Ensembl: The Ensembl project produces genome databases for vertebrates and other eukaryotic species, and makes this information freely available online.[27]
- MapView: The Map Viewer provides a wide variety of genome mapping and sequencing data.[28]
- VISTA is a comprehensive suite of programs and databases for comparative analysis of genomic sequences. It was built to visualize the results of comparative analysis based on DNA alignments. The presentation of comparative data generated by VISTA can easily suit both small and large scale of data.[29]
An advantage of using online tools is that these websites are being developed and updated constantly. There are many new settings and content can be used online to improve efficiency.[25]
Applications
Agriculture
Agriculture is a field that reaps the benefits of comparative genomics. Identifying the loci of advantageous genes is a key step in breeding crops that are optimized for greater yield, cost-efficiency, quality, and disease resistance. For example, one genome wide association study conducted on 517 rice landraces revealed 80 loci associated with several categories of agronomic performance, such as grain weight, amylose content, and drought tolerance. Many of the loci were previously uncharacterized.[30] Not only is this methodology powerful, it is also quick. Previous methods of identifying loci associated with agronomic performance required several generations of carefully monitored breeding of parent strains, a time consuming effort that is unnecessary for comparative genomic studies.[31]
Medicine
The medical field also benefits from the study of comparative genomics. Vaccinology in particular has experienced useful advances in technology due to genomic approaches to problems. In an approach known as reverse vaccinology, researchers can discover candidate antigens for vaccine development by analyzing the genome of a pathogen or a family of pathogens.[32] Applying a comparative genomics approach by analyzing the genomes of several related pathogens can lead to the development of vaccines that are multiprotective. A team of researchers employed such an approach to create a universal vaccine for Group B Streptococcus, a group of bacteria responsible for severe neonatal infection.[33] Comparative genomics can also be used to generate specificity for vaccines against pathogens that are closely related to commensal microorganisms. For example, researchers used comparative genomic analysis of commensal and pathogenic strains of E. coli to identify pathogen specific genes as a basis for finding antigens that result in immune response against pathogenic strains but not commensal ones.[34]
Research
Comparative genomics also opens up new avenues in other areas of research. As DNA sequencing technology has become more accessible, the number of sequenced genomes has grown. With the increasing reservoir of available genomic data, the potency of comparative genomic inference has grown as well. A notable case of this increased potency is found in recent primate research. Comparative genomic methods have allowed researchers to gather information about genetic variation, differential gene expression, and evolutionary dynamics in primates that were indiscernible using previous data and methods.[35] The Great Ape Genome Project used comparative genomic methods to investigate genetic variation with reference to the six great ape species, finding healthy levels of variation in their gene pool despite shrinking population size.[36] Another study showed that patterns of DNA methylation, which are a known regulation mechanism for gene expression, differ in the prefrontal cortex of humans versus chimps, and implicated this difference in the evolutionary divergence of the two species.[37]
See also
- Molecular evolution
- Comparative anatomy
- Homology
- Sequence mining
- Alignment-free sequence analysis
- DNA Patterns analysis
References
- ↑ Darling AE, Miklós I, Ragan MA (2008). "Dynamics of Genome Rearrangement in Bacterial Populations". PLOS Genetics. 4 (7): e1000128. doi:10.1371/journal.pgen.1000128.

- 1 2 3 Touchman, J. (2010). "Comparative Genomics". Nature Education Knowledge. 3 (10): 13. Retrieved 2014-01-02.
- 1 2 Xia, X. (2013). Comparative Genomics. Heidelberg: Springer. ISBN 978-3-642-37145-5.
- 1 2 Russel, P.J.; Hertz, P.E.; McMillan, B. (2011). Biology: The Dynamic Science (2nd ed.). Belmont, CA: Brooks/Cole. pp. 409–410.
- ↑ Primrose, S.B.; Twyman, R.M. (2003). Principles of Genome Analysis and Genomics (3rd ed.). Malden, MA: Blackwell Publishing.
- ↑ Hardison, R.C. (2003). "Comparative genomics". PLoS Biology. 1 (2): e58. doi:10.1371/journal.pbio.0000058. PMC 261895
 . PMID 14624258.
. PMID 14624258. 
- ↑ Ellegren, H. (2008). "Comparative genomics and the study of evolution by natural selection". Molecular Ecology. 17 (21): 4586–4596. doi:10.1111/j.1365-294X.2008.03954.x.
- 1 2 3 4 Koonin, E.V.; Galperin, M.Y. (2003). Sequence - Evolution - Function: Computational approaches in comparative genomics. Dordrecht: Springer Science+Business Media.
- 1 2 Hu, B.; Xie, G.; Lo, C.-C.; Starkenburg, S. R.; Chain, P. S. G. (2011). "Pathogen comparative genomics in the next-generation sequencing era: genome alignments, pangenomics and metagenomics". Briefings in Functional Genomics. 10 (6): 322–333. doi:10.1093/bfgp/elr042.
- ↑ Argos, P.; Kamer, G.; Nicklin, M.J.; Wimmer, E. (1984). "Similarity in gene organization and homology between proteins of animal picornaviruses and a plant comovirus suggest common ancestry of these virus families". Nucleic Acids Research. 12 (18): 7251–7267. doi:10.1093/nar/12.18.7251.
- ↑ McGeoch, D.J.; Davison, A.J. (1986). "DNA sequence of the herpes simplex virus type 1 gene encoding glycoprotein gH, and identification of homologues in the genomes of varicella-zoster virus and Epstein-Barr virus". Nucleic Acids Research. 14 (10): 4281–4292. doi:10.1093/nar/14.10.4281.
- ↑ Fleischmann R, Adams M, White O, Clayton R, Kirkness E, Kerlavage A, Bult C, Tomb J, Dougherty B, Merrick J (1995). "Whole-genome random sequencing and assembly of Haemophilus influenzae Rd". Science. 269 (5223): 496–512. Bibcode:1995Sci...269..496F. doi:10.1126/science.7542800. PMID 7542800.
- ↑ Fraser, Claire M.; et al. (1995). "The Minimal Gene Complement of Mycoplasma genitalium". Science. 270 (5235): 397–404. Bibcode:1995Sci...270..397F. doi:10.1126/science.270.5235.397. PMID 7569993.
- ↑ A. Goffeau; B. G. Barrell; H. Bussey; R. W. Davis; B. Dujon; H. Feldmann; F. Galibert; J. D. Hoheisel; C. Jacq; M. Johnston; E. J. Louis; H. W. Mewes; Y. Murakami; P. Philippsen; H. Tettelin; S. G. Oliver (1996). "Life with 6000 genes". Science. 274 (5287): 546, 563–567. Bibcode:1996Sci...274..546G. doi:10.1126/science.274.5287.546. PMID 8849441.
- ↑ The C. elegans Sequencing Consortium (1998). "Genome sequence of the nematode C. elegans: A platform for investigating biology". Science. 282 (5396): 2012–2018. doi:10.1126/science.282.5396.2012. PMID 9851916.
- ↑ Adams MD, Celniker SE, Holt RA, et al. (2000). "The genome sequence of Drosophila melanogaster". Science. 287 (5461): 2185–95. Bibcode:2000Sci...287.2185.. doi:10.1126/science.287.5461.2185. PMID 10731132.
- ↑ Rubin, G.; Yandell, M.; Wortman, J.; Gabor Miklos, G.; Nelson, C.; Hariharan, I.; Fortini, M.; Li, P.; Apweiler, R.; Fleischmann, W.; Cherry, J. M.; Henikoff, S.; Skupski, M. P.; Misra, S.; Ashburner, M.; Birney, E.; Boguski, M. S.; Brody, T.; Brokstein, P.; Celniker, S. E.; Chervitz, S. A.; Coates, D.; Cravchik, A.; Gabrielian, A.; Galle, R. F.; Gelbart, W. M.; George, R. A.; Goldstein, L. S.; Gong, F.; Guan, P. (2000). "Comparative genomics of the eukaryotes". Science. 287 (5461): 2204–2215. Bibcode:2000Sci...287.2204.. doi:10.1126/science.287.5461.2204. PMC 2754258
 . PMID 10731134.
. PMID 10731134. - ↑ Serafim Batzoglou, Lior Pachter, Jill Mesirov, Bonnie Berger and Eric Lander (2000). "Human and mouse gene structure: comparative analysis and application to exon prediction". Genome Research. 10: 950–958. doi:10.1101/gr.10.7.950.

- ↑ Ureta-Vidal, A.; Ettwiller, L.; Birney, E. (2003). "Comparative genomics: Genome-wide analysis in metazoan eukaryotes". Nature Reviews Genetics. 4 (4): 251–262. doi:10.1038/nrg1043. PMID 12671656.
- ↑ Stein LD; et al. (2003). "The genome sequence of Caenorhabditis briggsae: a platform for comparative genomics". PLoS Biology. 1 (2): E45. doi:10.1371/journal.pbio.0000045. PMC 261899
 . PMID 14624247.
. PMID 14624247. 
- ↑ "Newly Sequenced Worm a Boon for Worm Biologists". PLoS Biology. 1 (2): e4–e4. 2003. doi:10.1371/journal.pbio.0000044.

- ↑ Cristianini N, Hahn M (2006). Introduction to Computational Genomics. Cambridge University Press. ISBN 0-521-67191-4.
- ↑ Pratas, D; Silva, R; Pinho, A; Ferreira, P (May 18, 2015). "An alignment-free method to find and visualise rearrangements between pairs of DNA sequences.". Scientific Reports (Nature Publishing Group). 5: 10203. Bibcode:2015NatSR...5E0203P. doi:10.1038/srep10203. PMID 25984837.
- ↑ Via, Allegra; Javier De Las Rivas; Teresa K. Attwood; David Landsman; Michelle D. Brazas; Jack A. M. Leunissen; Anna Tramontano; Maria Victoria Schneider (2011-10-27). "Ten Simple Rules for Developing a Short Bioinformatics Training Course". PLoS Comput Biol. 7 (10): e1002245. Bibcode:2011PLSCB...7E2245V. doi:10.1371/journal.pcbi.1002245. Retrieved 2011-12-03.

- 1 2 3 Bergman NH, ed. (2007). Comparative Genomics: Volumes 1 and 2. Totowa (NJ): Humana Press. ISBN 978-193411-537-4. PMID 21250292.
- ↑ "UCSC Browser".
- ↑ "Ensembl Genome Browser".
- ↑ "Map Viewer".
- ↑ "VISTA tools".
- ↑ Huang XH; et al. (2010). "Genome-wide association studies of 14 agronomic traits in rice landraces". Nature Genetics. 42 (11): 961-U76. doi:10.1038/ng.695.

- ↑ Morrell PL, Buckler ES, Ross-Ibara J (2012). "Crop genomics: advances and applications". Nature Reviews Genetics. 13 (2): 85–96. doi:10.1038/nrg3097.

- ↑ Seib KL, Zhao X, Rappuoli R (2012). "Developing vaccines in the era of genomics: a decade of reverse vaccinology". Clinical Microbiology and Infection. 18 (SI): 109–116. doi:10.1111/j.1469-0691.2012.03939.x.

- ↑ Maione D; et al. (2005). "Identification of a Universal Group B Streptococcus Vaccine by Multiple Genome Screen". Science. 309 (5731): 148–150. Bibcode:2005Sci...309..148M. doi:10.1126/science.1109869.

- ↑ Rasco DA; et al. (2008). "The pangenome structure of Escherichia coli: Comparative genomic analysis of E-coli commensal and pathogenic isolates". Journal of Bacteriology. 190 (20): 6881–6893. doi:10.1128/JB.00619-08.

- ↑ Rodgers J, Gibbs RA (2014). "APPLICATIONS OF NEXT-GENERATION SEQUENCING Comparative primate genomics: emerging patterns of genome content and dynamics". Nature Reviews Genetics. 15 (5): 347–359. doi:10.1038/nrg3707.

- ↑ Prado-Martinez J; et al. (2013). "Great ape genetic diversity and population history". Nature. 499 (7459): 471–475. Bibcode:2013Natur.499..471P. doi:10.1038/nature12228.

- ↑ Zeng J, Konopa G, Hunt BG, Preuss TM, Geschwind D, Yi SV (2012). "Divergent Whole-Genome Methylation Maps of Human and Chimpanzee Brains Reveal Epigenetic Basis of Human Regulatory Evolution". The American Journal of Human Genetics. 91 (3): 455–465. doi:10.1016/j.ajhg.2012.07.024.

Further reading
- Bergman NH, ed. (2007). Comparative Genomics: Volumes 1 and 2. Totowa (NJ): Humana Press. ISBN 978-193411-537-4. PMID 21250292.
- Kellis M, Patterson N, Endrizzi M, Birren B, Lander E (2003-05-15). "Sequencing and comparison of yeast species to identify genes and regulatory elements". Nature. 423 (6937): 241–254. Bibcode:2003Natur.423..241K. doi:10.1038/nature01644. PMID 12748633.
- Cliften P, Sudarsanam P, Desikan A (2003-07-04). "Finding functional features in Saccharomyces genomes by phylogenetic footprinting". Science. 301 (5629): 71–76. Bibcode:2003Sci...301...71C. doi:10.1126/science.1084337. PMID 12775844.
- Boffeli D, McAuliffe J, Ovcharenko D, Lewis KD, Ovcharenko I, Pachter L, Rubin EM (2003). "Phylogenetic shadowing of primate sequences to find functional regions of the human genome". Science. 299 (5611): 1391–1394. doi:10.1126/science.1081331. PMID 12610304.
- Dujon B; et al. (2004-07-01). "Genome evolution in yeasts". Nature. 430 (6995): 35–44. Bibcode:2004Natur.430...35D. doi:10.1038/nature02579. PMID 15229592.
- Filipski A, Kumar S (2005). "Comparative genomics in eukaryotes". In T.R. Gregory. The Evolution of the Genome. San Diego: Elsevier. pp. 521–583.
- Gregory TR, DeSalle R (2005). "Comparative genomics in prokaryotes". In T.R. Gregory. The Evolution of the Genome. San Diego: Elsevier. pp. 585–675.
- Xie X; Lu J. Kulbokas EJ; Golub T; Mootha V; Lindblad-Toh K; Lander E; Kellis M (2005). "Systematic discovery of regulatory motifs in human promoters and 3' UTRs by comparison of several mammals". Nature. 434 (7031): 338–345. Bibcode:2005Natur.434..338X. doi:10.1038/nature03441. PMC 2923337
 . PMID 15735639.
. PMID 15735639. - Champ PC, Binnewies TT, Nielsen N, Zinman G, Kiil K, Wu H, Bohlin J, Ussery DW (2006). "Genome update: purine strand bias in 280 bacterial chromosomes". Microbiology. 152 (3): 579–583. doi:10.1099/mic.0.28637-0.
- Kumar L, Breakspear A, Kistler A, Ma LJ, Xie X (2010). "Systematic discovery of regulatory motifs in Fusarium graminearum by comparing four Fusarium genomes". BMC Genomics. 11: 208. doi:10.1186/1471-2164-11-208. PMC 2853525
 . PMID 20346147.
. PMID 20346147. 
- Serafim Batzoglou, Lior Pachter, Jill Mesirov, Bonnie Berger and Eric Lander (2000). "Human and mouse gene structure: comparative analysis and application to exon prediction". Genome Research. 10: 950–958. doi:10.1101/gr.10.7.950.

External links
- Genomes OnLine Database (GOLD)
- Genome News Network
- JCVI Comprehensive Microbial Resource
- Pathema: A Clade Specific Bioinformatics Resource Center
- CBS Genome Atlas Database
- The UCSC Genome Browser
- The U.S. National Human Genome Research Institute
- Ensembl The Ensembl Genome Browser
- Genolevures, comparative genomics of the Hemiascomycetous yeasts
- Phylogenetically Inferred Groups (PhIGs), a recently developed method incorporates phylogenetic signals in building gene clusters for use in comparative genomics.
- Metazome, a resource for the phylogenomic exploration and analysis of Metazoan gene families.
- IMG The Integrated Microbial Genomes system, for comparative genome analysis by the DOE-JGI.
- Dcode.org Dcode.org Comparative Genomics Center.
- SUPERFAMILY Protein annotations for all completely sequenced organisms
- Comparative Genomics
- Blastology and Open Source: Needs and Deeds
- Alignment-free comparative Genomics tool