Cyanamide
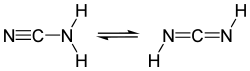 | |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC names
Cyanamide, aminomethanenitrile | |||
| Other names
Amidocyanogen, carbamonitrile, carbimide, carbodiimide, cyanoamine, cyanoazane, N-cyanoamine, cyanogenamide, cyanogen amide, cyanogen nitride, diiminomethane, hydrogen cyanamide, methane diimide | |||
| Identifiers | |||
| 420-04-2 | |||
| 3D model (Jmol) | Interactive image | ||
| ChEBI | CHEBI:16698 | ||
| ChEMBL | ChEMBL56279 | ||
| ChemSpider | 9480 | ||
| DrugBank | DB02679 | ||
| ECHA InfoCard | 100.006.358 | ||
| EC Number | 206-992-3 | ||
| KEGG | D00123 | ||
| PubChem | 9864 | ||
| RTECS number | GS5950000 | ||
| UNII | 21CP7826LC | ||
| UN number | 2811 | ||
| |||
| |||
| Properties | |||
| CH2N2 | |||
| Molar mass | 42.040 g/mol | ||
| Appearance | Crystalline solid | ||
| Density | 1.28 g/cm3 | ||
| Melting point | 44 °C (111 °F; 317 K) | ||
| Boiling point | 260 °C (500 °F; 533 K) (decomposes) 83 °C at 6.7 Pa 140 °C at 2.5 kPa | ||
| 85 g/100 ml (25 °C) | |||
| Solubility in organic solvents | soluble | ||
| Hazards | |||
| Safety data sheet | ICSC 0424 | ||
| EU classification (DSD) |
Toxic (T) | ||
| R-phrases | R20, R25, R27, R36/38, R43 | ||
| S-phrases | (S1/2), S3, S22, S36/37, S45 | ||
| NFPA 704 | |||
| Flash point | 141 °C (286 °F; 414 K) | ||
| US health exposure limits (NIOSH): | |||
| PEL (Permissible) |
none[1] | ||
| REL (Recommended) |
TWA 2 mg/m3 | ||
| IDLH (Immediate danger) |
N.D.[1] | ||
| Related compounds | |||
| Related compounds |
Calcium cyanamide | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| | |||
| Infobox references | |||
Cyanamide is an organic compound with the formula CN2H2. This white solid is widely used in agriculture and the production of pharmaceuticals and other organic compounds. It is also used as an alcohol deterrent drug in Canada, Europe and Japan. The molecule features a nitrile group attached to an amino group. Derivatives of this compound are also referred to as cyanamides, the most common being calcium cyanamide (CaCN2).
Tautomers and self-condensations
Containing both a nucleophilic and electrophilic site within the same molecule, cyanamide undergoes various reactions with itself. Cyanamide exists as two tautomers, one with the connectivity NCNH2 and the other with the formula HNCNH ("diimide" tautomer). The NCNH2 form dominates, but in a few reactions (e.g. silylation) the diimide form appears to be important.
Cyanamide dimerizes to give 2-cyanoguanidine (dicyandiamide). This decomposition process is disfavored by acids and is inhibited by low temperatures. The cyclic trimer is called melamine.
Production
Cyanamide is produced by hydrolysis of calcium cyanamide, which in turn is prepared from calcium carbide via the Frank-Caro process.
- CaCN2 + H2O + CO2 → CaCO3 + H2NCN
The conversion is conducted on slurries. Consequently, most commercial cyanamide is sold as an aqueous solution.
Reactions and uses
Cyanamide can be regarded as a functional single carbon fragment which can react as an electrophile or nucleophile. The main reaction exhibited by cyanamide involves additions of compounds containing an acidic proton. Water, hydrogen sulfide, and hydrogen selenide react with cyanamide to give urea, thiourea, and selenourea, respectively:
- H2NCN + H2E → H2NC(E)NH2 (E = O, S, Se)
In this way, cyanamide behaves as a dehydration agent and thus can induce condensation reactions. Alcohols, thiols, and amines react analogously to give alkylisoureas, "pseudothioureas," and guanidines. The anti-ulcer drug cimetidine is generated using such reactivity. Related reactions exploit the bifunctionality of cyanamide to give heterocycles, and this latter reactivity is the basis of several pharmaceutical syntheses such as the aminopyrimidine imatinib) and agrichemicals Amitrol and hexazinone. The hair-loss treatment minoxidil and the anthelmintics albendazole, flubendazole, and mebendazole feature 2-aminoimidazole substructures derived from cyanamide.[2] Cyanamide is also used in the synthesis of other pharmaceutical drugs including tirapazamine, etravirine, revaprazan, and dasantafil.
The cyanamide anion has the character of a pseudo chalkogene, cyanamid can therefore be regarded as analogue to water or hydrogen sulfide.
A convenient method for the preparation of secondary amines which are not contaminated with primary or tertiary amines is the reaction of cyanamide with alkyl halides to N,N-dialkylcyanamides which can easily be hydrolyzed to dialkylamines and then decarboxylated.[3] Cyanamide adds itself in the presence of N-bromosuccinimide to olefinic double bonds. The addition product is converted by bases to N-Cyanaziridine,[4] cyclized in the presence of acids to imidazolines, which can be further reacted to vicinale diamines by alkaline cleavage.[5]
Cyanamid is also a versatile synthetic building block for heterocycles: it forms 2-aminobenzimidazole with 1,2-diaminobenzene[6] and it forms with the readily available cyclic enamine 4-(1-cyclohexenyl)morpholine[7] and an elemental sulfur a 2-aminothiazole in good yields.[8]
Sodium dicyanamide is available in good yield and high purity from cyanamid and cyanogen chloride,[9] which is suitable as an intermediate for the synthesis of active pharmaceutical ingredients.[10] A guanidino group is introduced by reaction of cyanamide with sarcosine In the industrial synthesis of creatine:[11]
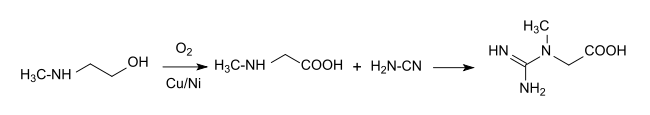
This synthesis route mostly avoids problematic impurities like chloroacetic acid, iminodiacetic acid or dihydrotriazine that occur in other routes. The physiological precursor guanidinoacetic is obtained analogously by reacting cyanamide with glycine.
Cyanamide is a common agricultural rest-breaking agent applied in spring to stimulate uniform opening of buds, early foliation and bloom. Cyanamide can effectively compensate for the moderate lack of chilling units accumulated in the previous autumn and save the harvest that would otherwise be lost. It is particularly effective for woody plants such as berries, grapes, apples, peaches and kiwifruit. Overdosage, high concentration and error in timing of application can damage the buds (especially of peach trees).[12] A 50% aqueous solution of cyanamide is also used as a biocide (disinfectant) particularly in pig farming, because it effectively kills salmonella and schigella and fights flys in all stages of development.[13]
Since the mid-1960s there are methods to stabilize cyanamid in order to make it available on an industrial scale. Due to the strong affinity towards self-condensation in alkaline media (see above) solutions of cyanamide are stabilized by the addition of 0.5 wt% of NaH2PO4 as buffer. Solid cyanamid is produced by careful evaporation of the solvent and subsequent addition of a hydrolysis labil ester of methanoic acid. The ester absorbs traces of moisture (suppression of urea formation), neutralizes alkalinity (ammonia) and continually releases small amounts of methanoic acid.[14]
Environmental aspects
Cyanamide degrades via hydrolysis to urea, an excellent fertilizer. Fungi, like Myrothecium verrucaria, accelerate this process utilizing the enzyme cyanamide hydratase.[15]
Safety
Cyanamide has a modest toxicity in humans.[16] Workplace exposure to hydrogen cyanamide sprays or exposure in people living in the vicinity of spraying have been reported as causing respiratory irritation, contact dermatitis, headache, and gastrointestinal symptoms of nausea, vomiting, or diarrhea.[16]
References
- 1 2 "NIOSH Pocket Guide to Chemical Hazards #0160". National Institute for Occupational Safety and Health (NIOSH).
- ↑ Thomas Güthner; Bernd Mertschenk (2006). "Cyanamides". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a08_139.pub2.
- ↑ Jonczyk, A.; Ochal, Z.; Makosza, M.: Reactions of Organic Anions; LXXXV1. Catalytic Two-Phase Alkylation of Cyanamide in Synthesis, 1978, 882–883, doi:10.1055/s-1978-24922.
- ↑ Ponsold, K.; Ihn, W.: Die Addition von cyanamid und Halogen an Olefine ein neues Verfahren zur Darstellung von vic.-Halogencyanaminen und Aziridinen in Tetrahedron Lett. 11 (1970) 1125–1128, doi:10.1016/S0040-4039(01)97925-0.
- ↑ H. Kohn, Sang Hun Jung, J. Am. Chem. Soc., 105, S. 4106 (1983), DOI:10.1021/ja00350a068.
- ↑ Angew. Chem. Int. Ed. 12, S. 841 (1973), DOI:10.1002/anie.197308411.
- ↑ S. Hünig, E. Lücke, and W. Brenninger (1961). "1-Morpholino-1-Cyclohexene". Org. Synth.: 65. doi:10.15227/orgsyn.041.0065.
- ↑ K. Gewald, H. Spies, R. Mayer, J. Prakt. Chem., 312, S. 776 (1970), DOI:10.1002/prac.19703120507.
- ↑ Verfahren zur Herstellung von Natrium-Dicyanamid, veröffentlicht am 10. August 2000, Anmelder: SKW Trostberg AG.
- ↑ Lonza: Natriumdicyanamid
- ↑ Deutsche Offenlegungsschrift DE-OS 10 2006 016 227 A1, Offenlegungsdatum: 11. Oktober 2007, Anmelder: Degussa GmbH.
- ↑ Powell, A. (1999). Action Program for Dormex Application on Peaches. Auburn University. Retrieved 2010-05-24.
- ↑ AlzChem: ALZOGUR.
- ↑ Wehrstedt, K.-D.; Wildner, W.; Güthner, T.; Holzrichter, K.; Mertschenk, B.; Ulrich, A.: Safe transport of cyanamide in J. Hazard. Mat. 170 (2009) 829–835, doi:10.1016/j.jhazmat.2009.05.043.
- ↑ Stransky H and Amberger A (1973). "Isolation and properties of a cyanamide hydratase (EC 4.2.1) from Myrothecium verrucaria". Z. Pflanzenphysiol. 70: 74–87, DOI:10.1016/S0044-328X(73)80049-2.
- 1 2 Schep L, Temple W, Beasley M (January 2009). "The adverse effects of hydrogen cyanamide on human health: an evaluation of inquiries to the New Zealand National Poisons Centre". Clinical Toxicology. Philadelphia, PA. 472 (1): 58–60. doi:10.1080/15563650802459254. PMID 18951270.
External links
- International Chemical Safety Card 0424
- "NIOSH Pocket Guide to Chemical Hazards #0160". National Institute for Occupational Safety and Health (NIOSH).
- OSHA guideline for cyanamide
_3D_spacefill.png)
_3D_spacefill.png)

