Endoreduplication
Endoreplication (also referred to as endoreduplication or polytenization) is replication of the nuclear genome in the absence of cell division, which leads to elevated nuclear gene content and polyploidy. Endoreplication can be understood simply as a variant form of the mitotic cell cycle (G1-S-G2-M) in which mitosis is aborted prior to cytokinesis or circumvented entirely, due in part to modulation of cyclin-dependent kinase (CDK) activity.[1][2] Examples of endoreplication characterized in arthropod, mammalian, and plant species suggest that it is a universal developmental mechanism responsible for the differentiation and morphogenesis of cell types that fulfill an array of biological functions.[1][2] While endoreplication is often limited to specific cell types in animals, it is considerably more widespread in plants, such that polyploidy can be detected in the majority of plant tissues.[3]
Examples in nature
Endoreplicating cell types that have been studied extensively in model organisms
| Organism | Cell Type | Biological Function | Citation |
|---|---|---|---|
| fly | larval tissues (incl. salivary glands) | secretion, embryogenesis | [4] |
| fly | ovarian follicle, nurse cells | nourishment, protection of oocytes | [5] |
| rodent | megakaryocyte | platelet formation | [6] |
| rodent | trophoblast giant cell | placental development, nourishment of embryo | [7] |
| plant | trichome | defense from herbivory, homeostasis | [8] |
| plant | leaf epidermal cell | leaf size, structure | [9] |
| plant | endosperm | nourishment of embryo | [10] |
| nematode | hypodermis | secretion, body size | [11] |
Endocycling vs. endomitosis
Endoreplication typically results in elevated cellular DNA content, but the manner in which the genetic material is configured varies depending on whether mitotic events are allowed to occur.[1][2] Endocycling is a form of endoreplication in which the cell largely avoids mitosis and duplicated chromatids remain physically associated. Repeated rounds of endocycling can lead to the production of polytene chromosomes in which sister chromatids are tightly configured in parallel arrays. In contrast, endomitosis is a form of endoreplication in which cells undergo aspects of mitosis but fail to execute telophase and/or cytokinesis. Duplicated chromosomes produced by endomitosis exist as discrete units in a single polyploid nucleus or may be packaged into separate nuclei, depending on the phase at which mitosis is aborted. In certain instances (e.g. trophoblast giant cells), the distinction between endocycling and endomitosis is clouded, as the cell undergoes early mitotic events such as chromosome condensation that tend to diminish the physical association among homologous chromosomes. It should also be noted that endocycling does not always involve replication of the entire genome, and that certain regions are often replicated more frequently than others.

Biological significance
Based on the wide array of cell types in which endoreplication occurs, a variety of hypotheses have been generated to explain the functional importance of this phenomenon.[1][2] Unfortunately, experimental evidence to support these conclusions is somewhat limited:
Cell/Organism Size: Cell ploidy often correlates with cell size,[9][11] and in some instances, disruption of endoreplication results in diminished cell and tissue size [12] suggesting that endoreplication may serve as a mechanism for tissue growth. Relative to mitosis, endoreplication does not require cytoskeletal rearrangement or the production of new cell membrane and it often occurs in cells that have already differentiated. As such it may represent an energetically efficient alternative to cell proliferation among differentiated cell types that can no longer afford to undergo mitosis.[13] While evidence establishing a connection between ploidy and tissue size is prevalent in the literature, contrary examples also exist.[14]
Cell Differentiation: In developing plant tissues the transition from mitosis to endoreplication often coincides with cell differentiation and morphogenesis.[14] However it remains to be determined whether endoreplication and polypoidy contribute to cell differentiation or vice versa. Interestingly, targeted inhibition of endoreplication in trichome progenitors results in the production of multicellular trichomes that exhibit relatively normal morphology, but ultimately dedifferentiate and undergo absorption into the leaf epidermis.[15] This result suggests that endoreplication and polyploidy may be required for the maintenance of cell identity.
Oogenesis and Embryonic Development: Endoreplication is commonly observed in cells responsible for the nourishment and protection of oocytes and embryos. It has been suggested that increased gene copy number might allow for the mass production of proteins required to meet the metabolic demands of embryogenesis and early development.[1] Consistent with this notion, mutation of the Myc oncogene in Drosophila follicle cells results in reduced endoreplication and abortive oogenesis.[16] However, reduction of endoreplication in maize endosperm has limited effect on the accumulation of starch and storage proteins, suggesting that the nutritional requirements of the developing embryo may involve the nucleotides that comprise the polyploid genome rather than the proteins it encodes.[17]
Buffering the Genome: Another intriguing hypothesis is that endoreplication buffers against DNA damage and mutation because it provides extra copies of important genes.[1] However, this notion is purely speculative and there is limited evidence to the contrary. For example, analysis of polyploid yeast strains suggests that they are more sensitive to radiation than diploid strains.[18]
Stress Response: Research in plants suggests that endoreplication may also play a role in modulating stress responses. By manipulating expression of E2fe (a repressor of endocycling in plants), researchers were able to demonstrate that increased cell ploidy lessens the negative impact of drought stress on leaf size.[19] Given that the sessile lifestyle of plants necessitates a capacity to adapt to environmental conditions, it is appealing to speculate that widespread polyploidization contributes to their developmental plasticity
Genetic control of endoreplication
The best-studied example of a mitosis-to-endocycle transition occurs in Drosophila follicle cells and is activated by Notch signaling.[20] Entry into endocycles involves modulation of mitotic and S-phase cyclin-dependent kinase (CDK) activity.[21] Inhibition of M-phase CDK activity is accomplished via transcriptional activation of Cdh/fzr and repression of the G2-M regulator string/cdc25.[21][22] Cdh/fzr is responsible for activation of the anaphase-promoting complex (APC) and subsequent proteolysis of the mitotic cyclins. String/cdc25 is a phosphatase that stimulates mitotic cyclin-CDK complex activity. Upregulation of S-phase CDK activity is accomplished via transcriptional repression of the inhibitory kinase dacapo. Together, these changes allow for the circumvention of mitotic entry, progression through G1, and entry into S-phase. The induction of endomitosis in mammalian megakaryocytes involves activation of the c-mpl receptor by the thrombopoietin (TPO) cytokine and is mediated by ERK1/2 signaling.[23] As with Drosophila follicle cells, endoreplication in megakaryocytes results from activation of S-phase cyclin-CDK complexes and inhibition of mitotic cyclin-CDK activity.[24][25]
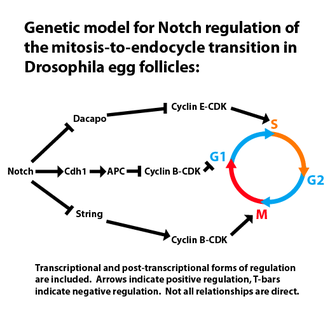
Entry into S-phase during endoreplication (and mitosis) is regulated through the formation of a prereplicative complex (pre-RC) at replication origins, followed by recruitment and activation of the DNA replication machinery. In the context of endoreplication these events are facilitated by an oscillation in cyclin E-Cdk2 activity. Cyclin E-Cdk2 activity drives the recruitment and activation of the replication machinery,[26] but it also inhibits pre-RC formation,[27] presumably to ensure that only one round of replication occurs per cycle. Failure to maintain control over pre-RC formation at replication origins results in a phenomenon known as “rereplication” which is common in cancer cells.[2] The mechanism by which cyclin E-Cdk2 inhibits pre-RC formation involves downregulation of APC-Cdh1-mediated proteolysis and accumulation of the protein Geminin, which is responsible for sequestration of the pre-RC component Cdt1.[28][29]
Oscillations in Cyclin E-Cdk2 activity are modulated via transcriptional and post-transcriptional mechanisms. Expression of cyclin E is activated by E2F transcription factors that were shown to be required for endoreplication.[30][31][32] Recent work suggests that observed oscillations in E2F and cyclin E protein levels result from a negative-feedback loop involving Cul4-dependent ubiquitination and degradation of E2F.[33] Post-transcriptional regulation of cyclin E-Cdk2 activity involves Ago/Fbw7-mediated proteolytic degradation of cyclin E [34][35] and direct inhibition by factors such as Dacapo and p57.[36][37] True endomitosis in the anther tapetum of the liliaceous plant Eremurus is described. The nuclear membrane does not disappear, but during metaphase the chromosomes are condensed, often considerably more than in normal mitosis. When the pollen mother cells (PMCs) go through the last premeiotic mitosis, the tapetal cells have one diploid nucleus which divides while the cell remains undivided. The two diploid nuclei may undergo an endomitosis and the resulting tetraploid nuclei a second endomitosis. An alternative pathway is an ordinary mitosis-again without cell division instead of one of the endomitotic cycles. The cytological picture in the tapetum is further complicated by restitution in anaphase and fusion of metaphase and anaphase groups during mitosis, processes which could give rise to cells with one, two, or three nuclei, instead of the expected two or four. No sign of the so-called "inhibited" mitosis is seen in these tapetal cells. When the PMCs are in leptotene-zygotene, very few tapetal nuclei are in endomitosis. When the PMCs have reached diplotene, almost 100% of cells which are not in interphase show an endomitotic stage.
Endoreplication and oncogenesis
Polyploidy and aneuploidy are common phenomena in cancer cells.[38] Given that oncogenesis and endoreplication likely involve subversion of common cell cycle regulatory mechanisms, a thorough understanding of endoreplication may provide important insights for cancer biology.
Premeiotic endomitosis in unisexual vertebrates
The unisexual salamanders (genus Ambystoma) are the oldest known unisexual vertebrate lineage, having arisen about 5 million years ago.[39] In these polyploid unisexual females, an extra premeiotic endomitotic replication of the genome, doubles the number of chromosomes.[40] As a result, the mature eggs that are produced subsequent to the two meiotic divisions have the same ploidy as the somatic cells of the adult female salamander. Synapsis and recombination during meiotic prophase I in these unisexual females is thought to ordinarily occur between identical sister chromosomes and occasionally between homologous chromosomes. Thus little, if any, genetic variation is produced. Recombination between homeologous chromosomes occurs rarely, if at all.[40] Since production of genetic variation is weak, at best, it is unlikely to provide a benefit sufficient to account for the maintenance of meiosis for millions of years. Perhaps the efficient recombinational repair of DNA damages at each generation provided by meiosis has been a sufficient advantage to maintain meiosis.[41]
References
- 1 2 3 4 5 6 Edgar BA; Orr-Weaver TL (2001). "Endoreplication cell cycles: more for less". Cell. 105 (3): 297–306. doi:10.1016/S0092-8674(01)00334-8. PMID 11348589.
- 1 2 3 4 5 Lee HO; Davidson JM; Duronio RJ (2008). "Endoreplication: polyploidy with purpose". Genes & Development. 23 (21): 2461–77. doi:10.1101/gad.1829209. PMC 2779750
 . PMID 19884253.
. PMID 19884253. - ↑ Galbraith DW; Harkins KR; Knapp S (1991). "Systemic Endopolyploidy in Arabidopsis thaliana". Plant Physiology. 96 (3): 985–9. doi:10.1104/pp.96.3.985. PMC 1080875
 . PMID 16668285.
. PMID 16668285. - ↑ Hammond MP; Laird CD (1985). "Control of DNA replication and spatial distribution of defined DNA sequences in salivary gland cells of Drosophila melanogaster". Chromosoma. 91 (3–4): 279–286. doi:10.1007/BF00328223. PMID 3920018.
- ↑ Hammond MP; Laird CD (1985). "Chromosome structure and DNA replication in nurse and follicle cells of Drosophila melanogaster". Chromosoma. 91 (3–4): 267–278. doi:10.1007/BF00328222. PMID 3920017.
- ↑ Ravid K; Lu J; Zimmet JM; Jones MR (2002). "Roads to polyploidy: The megakaryocyte example". Journal of Cell Physiology. 190: 7–20. doi:10.1002/jcp.10035.
- ↑ Cross JC (2005). "How to make a placenta: Mechanisms of trophoblast cell differentiation in mice-a review". Placenta. 26: S3–9. doi:10.1016/j.placenta.2005.01.015. PMID 15837063.
- ↑ Hulskamp M; Schnittger A; Folkers U (1999). "Pattern formation and cell differentiation: Trichomes in Arabidopsis as a genetic model system". International Review of Cytology. International Review of Cytology. 186: 147–178. doi:10.1016/S0074-7696(08)61053-0. ISBN 978-0-12-364590-6. PMID 9770299.
- 1 2 Melaragno JE; Mehrotra B; Coleman AW (1993). "Relationship between endopolyploidy and cell size in epidermal tissue of Arabidopsis". The Plant Cell. 5 (11): 1661–8. doi:10.2307/3869747. JSTOR 3869747.
- ↑ Sabelli PA; Larkins BA (2009). "The Development of Endosperm in Grasses". Plant Physiology. 149 (1): 14–26. doi:10.1104/pp.108.129437. PMC 2613697
 . PMID 19126691.
. PMID 19126691. - 1 2 Flemming AJ; Shen Z; Cunha A; Emmons SW; Leroi AM (2000). "Somatic polyploidization and cellular proliferation drive body size evolution in nematodes". PNAS. 97 (10): 5285–90. doi:10.1073/pnas.97.10.5285. PMC 25820
 . PMID 10805788.
. PMID 10805788. - ↑ Lozano E; Saez AG; Flemming AJ; Cunha A; Leroi AM (2006). "Regulation of growth by ploidy in Caenorhabditis elegans". Current Biology. 16 (5): 493–8. doi:10.1016/j.cub.2006.01.048. PMID 16527744.
- ↑ Kondorosi E; Roudier F; Gendreau E (2000). "Plant cell-size control: Growing by ploidy?". Current Opinion in Plant Biology. 3 (6): 488–492. doi:10.1016/S1369-5266(00)00118-7. PMID 11074380.
- 1 2 Inze D; De Veylder L (2006). "Cell cycle regulation in plant development". Annual Review of Genetics. 40: 77–105. doi:10.1146/annurev.genet.40.110405.090431. PMID 17094738.
- ↑ Bramsiepe J; Wester K; Weinl C; Roodbarkelari F; Kasili R; Larkin JC; Hulskamp M; Schnittger A (2010). Qu, Li-Jia, ed. "Endoreplication Controls Cell Fate Maintenance". PLOS Genetics. 6 (6): e1000996. doi:10.1371/journal.pgen.1000996. PMC 2891705
 . PMID 20585618.
. PMID 20585618. - ↑ Maines JZ; Stevens LM; Tong X; Stein D (2004). "Drosophila dMyc is required for ovary cell growth and endoreplication". Development. 131 (4): 775–786. doi:10.1242/dev.00932. PMID 14724122.
- ↑ Leiva-Neto JT; Grafi G; Sabelli PA; Dante RA; Woo YM; Maddock S; Gordon-Kamm WJ; Larkins BA (2004). "A Dominant Negative Mutant of Cyclin-Dependent Kinase A Reduces Endoreduplication but Not Cell Size or Gene Expression in Maize Endosperm". The Plant Cell. 16 (7): 1854–69. doi:10.1105/tpc.022178. PMC 514166
 . PMID 15208390.
. PMID 15208390. - ↑ Mortimer RK (1958). "Radiobiological and genetic studies on a polyploid series (haploid to hexaploid) of Saccharomyces cerevisiae". Radiation Research. 9 (3): 312–326. doi:10.2307/3570795. JSTOR 3570795. PMID 13579200.
- ↑ Cookson SJ; Radziejwoski A; Granier C (2006). "Cell and leaf size plasticity in Arabidopsis: what is the role of endoreplication?". Plant, Cell and Environment. 29 (7): 1273–83. doi:10.1111/j.1365-3040.2006.01506.x.
- ↑ Deng WM; Althauser C; Ruohala-Baker H (2001). "Notch-Delta signaling induces a transition from mitotic cell cycle to endocycle in Drosophila follicle cells:". Development. 128 (23): 4737–46. PMID 11731454.
- 1 2 Shcherbata HR; Althauser C; Findley SD; Ruohola-Baker H (2004). "The mitotic-to-endocycle switch inDrosophila follicle cells is executed by Notch-dependent regulation of G1/S, G2/M and M/G1 cell-cycle transitions". Development. 131 (13): 3169–81. doi:10.1242/dev.01172. PMID 15175253.
- ↑ Schaeffer V; Althauser C; Shcherbata HR; Deng WM; Ruohola-Baker H (2004). "Notch-dependent Fizzy-related/Hec1/Cdh1 expression is required for the mitotic-to-endocycle transition in Drosophila follicle cells". Current Biology. 14 (7): 630–6. doi:10.1016/j.cub.2004.03.040. PMID 15062106.
- ↑ Kaushansky K (2005). "The molecular mechanisms that control thrombopoiesis". The Journal of Clinical Investigation. 115 (12): 3339–47. doi:10.1172/JCI26674. PMC 1297257
 . PMID 16322778.
. PMID 16322778. - ↑ Garcia P; Cales C (1996). "Endoreplication in megakaryoblastic cell lines is accompanied by sustained expression of G1/S cyclins and downregulation of cdc25c". Oncogene. 13 (4): 695–703. PMID 8761290.
- ↑ Zhang Y; Wang Z; Ravid K (1996). "The cell cycle in polyploid megakaryocytes is associated with reduced activity of cyclin B1-dependent cdc2 kinase". Journal of Biological Chemistry. 271 (8): 4266–72. doi:10.1074/jbc.271.8.4266. PMID 8626773.
- ↑ Su TT; O'Farrell PH (1998). "Chromosome Association of Minichromosome Maintenance Proteins in Drosophila Endoreplication Cycles". Journal of Cell Biology. 140 (3): 451–460. doi:10.1083/jcb.140.3.451. PMC 2140170
 . PMID 9456309.
. PMID 9456309. - ↑ Arias EE; Walter JC (2004). "Strength in numbers: Preventing rereplication via multiple mechanisms in eukaryotic cells". Genes & Development. 21 (5): 497–518. doi:10.1101/gad.1508907. PMID 17344412.
- ↑ Narbonne-Reveau K; Senger S; Pal M; Herr A; Richardson HE; Asano M; Deak P; Lilly MA (2008). "APC/CFzr/Cdh1 promotes cell cycle progression during the Drosophila endocycle". Development. 135 (8): 1451–61. doi:10.1242/dev.016295. PMID 18321983.
- ↑ Zielke N; Querings S; Rottig C; Lehner C; Sprenger F (2008). "The anaphase-promoting complex/cyclosome (APC/C) is required for rereplication control in endoreplication cycles". Genes & Development. 22 (12): 1690–1703. doi:10.1101/gad.469108. PMC 2428065
 . PMID 18559483.
. PMID 18559483. - ↑ Duronio RJ; O'Farrell PH (1995). "Developmental control of the G1 to S transition in Drosophila: Cyclin E is a limiting downstream target of E2F". Genes & Development. 9 (12): 1456–68. doi:10.1101/gad.9.12.1456. PMID 7601350.
- ↑ Duronio RJ; O'Farrell PH; Xie JE; Brook A; Dyson N (1995). "The transcription factor E2F is required for S phase during Drosophila embryogenesis". Genes & Development. 9 (12): 1445–55. doi:10.1101/gad.9.12.1445. PMID 7601349.
- ↑ Duronio RJ; Bonnette PC; O'Farrell PH (1998). "Mutations of the Drosophila dDP, dE2F, and cyclin E Genes Reveal Distinct Roles for the E2F-DP Transcription Factor and Cyclin E during the G1-S Transition". Molecular and Cellular Biology. 18 (1): 141–151. PMC 121467
 . PMID 9418862.
. PMID 9418862. - ↑ Shibutani ST; de la Cruz AF; Tran V; Turbyfill WJ; Reis T; Edgar BA; Duronio RJ (2008). "Intrinsic negative cell cycle regulation provided by PIP box- and Cul4Cdt2-mediated destruction of E2f1 during S phase". Developmental Cell. 15 (6): 890–900. doi:10.1016/j.devcel.2008.10.003. PMC 2644461
 . PMID 19081076.
. PMID 19081076. - ↑ Koepp DM; Schaefer LK; Ye X; Keyomarsi K; Chu C; Harper JW; Elledge SJ (2001). "Phosphorylation-dependent ubiquitination of cyclin E by the SCFFbw7 ubiquitin ligase". Science. 294 (5540): 173–7. doi:10.1126/science.1065203. PMID 11533444.
- ↑ Moberg KH; Bell DW; Wahrer DC; Haber DA; Hariharan IK (2001). "Archipelago regulates cyclin E levels in Drosophila and is mutated in human cancer lines". Nature. 413 (6853): 311–6. doi:10.1038/35095068. PMID 11565033.
- ↑ de Nooij JC; Graber KH; Hariharan IK (2001). "Expression of cyclin-dependent kinase inhibitor Dacapo is regulated by cyclin E". Mechanisms of Development. 97 (1–2): 73–83. doi:10.1016/S0925-4773(00)00435-4. PMID 11025208.
- ↑ Ullah Z; Kohn MJ; Yagi R; Vassilev LT; DePamphilis ML (2008). "Differentiation of trophoblast stem cells into giant cells is triggered by p57/Kip2 inhibition of CDK1 activity". Genes & Development. 22 (21): 3024–36. doi:10.1101/gad.1718108. PMC 2577795
 . PMID 18981479.
. PMID 18981479. - ↑ Storchova Z; Pellman D (2004). "From polyploidy to aneuploidy, genome instability and cancer". Nature Reviews Molecular Cell Biology. 5 (1): 45–54. doi:10.1038/nrm1276. PMID 14708009.
- ↑ Bi K, Bogart JP (2010). "Time and time again: unisexual salamanders (genus Ambystoma) are the oldest unisexual vertebrates". BMC Evol. Biol. 10: 238. doi:10.1186/1471-2148-10-238. PMC 3020632
 . PMID 20682056.
. PMID 20682056. - 1 2 Bi K, Bogart JP (2010). "Probing the meiotic mechanism of intergenomic exchanges by genomic in situ hybridization on lampbrush chromosomes of unisexual Ambystoma (Amphibia: Caudata)". Chromosome Res. 18 (3): 371–82. doi:10.1007/s10577-010-9121-3. PMID 20358399.
- ↑ Harris Bernstein, Carol Bernstein and Richard E. Michod (2011). Meiosis as an Evolutionary Adaptation for DNA Repair. Chapter 19 in DNA Repair. Inna Kruman editor. InTech Open Publisher. DOI: 10.5772/25117 http://www.intechopen.com/books/dna-repair/meiosis-as-an-evolutionary-adaptation-for-dna-repair