Environmental impact of pharmaceuticals and personal care products

The environmental effect of pharmaceuticals and personal care products (PPCPs) is largely speculative. PPCPs are substances used by individuals for personal health or cosmetic reasons and the products used by agribusiness to boost growth or health of livestock. PPCPs have been detected in water bodies throughout the world. The effects of these chemicals on humans and the environment are not yet known, but to date there is no scientific evidence that they affect human health.[1] The term PPCPs contains as well environmental persistent pharmaceutical pollutants (EPPPs). The European Union summarizes pharmaceutical residues with the potential of contamination of water and soil together with other micropollutants under “priority substances”.[2]
Overview
Since the 1990s water contamination by pharmaceuticals has been an environmental issue of concern.[3] Most pharmaceuticals are deposited in the environment through human consumption and excretion, and are often filtered ineffectively by wastewater treatment plants which are not designed to manage them. Once in the water they can have diverse, subtle effects on organisms, although research is limited. Pharmaceuticals may also be deposited in the environment through improper disposal, runoff from sludge fertilizer and reclaimed wastewater irrigation, and leaky sewage.[3] In 2009 an investigative report by Associated Press concluded that U.S. manufacturers had legally released 271 million pounds of compounds used as drugs into the environment, 92 percent of which was the industrial chemicals phenol and hydrogen peroxide, which are also used an antiseptics. It could not distinguish between drugs released by manufacturers as opposed to the pharmaceutical industry. It also found that an estimated 250 million pounds of pharmaceuticals and contaminated packaging were discarded by hospitals and long-term care facilities.[4] In parallel, the European Union is the second biggest consumer in the world (24% of the world total) after the USA and in the majority of EU Member States, around 50% of unused human medicinal products is not collected to be discharged properly. In the EU between 30 and 90% of the orally administered dose is estimated to be excreted as active substance in the urine.[5]
The term environmental persistent pharmaceutical pollutants (EPPP) was suggested in the 2010 nomination of pharmaceuticals and environment as an emerging issue to Strategic Approach to International Chemicals Management (SAICM) by the International Society of Doctors for the Environment (ISDE).
Safe disposal
Depending on the source and ingredients, there are various ways in which the public can dispose of pharmaceutical and personal care products. In the case of pharmaceutical products, the most environmentally safe disposal method is to take advantage of a community drug take-back programs that collect drugs at a central location for proper disposal. Several local public health departments in the United States have initiated pharmaceutical take-back programs. In addition, the United States Drug Enforcement Administration (DEA) periodically promotes local take-back programs as well as a program called the National Take Back Initiative.[6] Currently, take back programs are funded by state or local health departments or are volunteer programs through pharmacies or health care providers. In recent years, the proposition that pharmaceutical companies should be responsible for their products “from the cradle to the grave,” has been gaining traction. This philosophy suggests that the manufacturers should fund the proper disposal of pharmaceutical products.[7] Take back programs should exist in every community, and if further information is required on the matter the city officials should be contacted.[8] The Environmental Protection Agency and the Office of National Drug Control Policy further emphasize that if no program is available to follow the subsequent measurements:
- take the prescription drugs out of their original containers
- mix drugs with cat litter or used coffee grounds
- place the mixture into a disposable container with a lid, such as a sealable bag
- cover up any personal identification with a black marker that is on the original pill containers
- place these containers in the bag with the mixture, seal them, and place them in the trash.
After these products are properly disposed, the process of treating them for minimizing environmental effect begins. Water treatment facilities use different processes in order to minimize or fully eliminate the amount of these pollutants. This is done by using sorption where suspended solids are removed by sedimentation.[9] Another method used is biodegradation, and through this method microorganisms, such as bacteria, feed or break down these pollutants thus eliminating them from the contaminated media.
Types
"Pharmaceuticals", or prescription and over-the-counter medications made for human use or veterinary or agribusiness purposes, are common PPCPs found in the environment.[10] Antibiotics, nutraceuticals (e.g., vitamins), supplements, and sexual enhancement drugs are contained in this group. "Personal care products" may include cosmetics, fragrances, menstrual care products, lotions, shampoos, soaps, toothpastes, and sunscreen. These products typically enter the environment when passed through or washed off the body and into the ground or sewer lines, or when disposed of in the trash, septic tank, or sewage system.[1]
Traces of illicit drugs can be found in waterways and may even be carried by money.[11]
Entry and presence in the environment
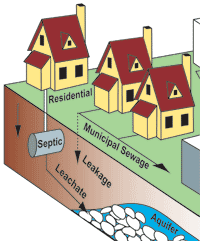
The use of pharmaceuticals and personal care products (PPCPs) is on the rise with an estimated increase from 2 billion to 3.9 billion annual prescriptions between 1999 and 2009 in the United States alone.[13] PPCPs enter into the environment through individual human activity and as residues from manufacturing, agribusiness, veterinary use, and hospital and community use. In Europe, the input of pharmaceutical residues via domestic waste water is estimated to be around 80% whereas 20% is coming from hospitals.[14] Individuals may add PPCPs to the environment through waste excretion and bathing as well as by directly disposing of unused medications to septic tanks, sewers, or trash. Because PPCPs tend to dissolve relatively easily and do not evaporate at normal temperatures, they often end up in soil and water bodies.
Some PPCPs are broken down or processed easily by a human or animal body and/or degrade quickly in the environment . However, others do not break down or degrade easily. The likelihood or ease with which an individual substance will break down depends on its chemical makeup and the metabolic pathway of the compound.[1]
A 2002 study by the U.S. Geological Survey found detectable quantities of one or more chemicals in 80 percent of a sampling of 139 susceptible streams in 30 states.[15] The most common pharmaceuticals detected were nonprescription drugs; detergents, fire retardants, pesticides, natural and synthetic hormones, and an assortment of antibiotics and prescription medications were also found.[16] A 2006 study found detectable concentrations of 28 pharmaceutical compounds in sewage treatment plant effluents, surface water, and sediment. The therapeutic classes included antibiotics, analgesics and anti-inflammatories, lipid regulators, beta-blockers, anti-convulsant, and steroid hormones. Although most chemical concentrations were detected at low levels (nano-grams/Liter (ng/L)), there are uncertainties that remain regarding the levels at which toxicity occurs and the risks of bioaccumulation of these pharmaceutical compounds.[17] A study published in late 2014 reported a spike in the levels of ecstasy, ketamine, caffeine and acetaminophen in nearby rivers coinciding with a Taiwanese youth event attended by around 600,000 people.[18] Besides the identified input from human medicine there appears diffuse pollution for example from pharmaceuticals used in agriculture, too. Investigations in Germany, France and Scotland showed traces of PPCPs upstream of waste water treatment plant effluents to rivers, too.[19]
Effects
Human
The scope of human exposure to pharmaceuticals and personal care products from the environment is a complex function of many factors. These factors include the concentrations, types, and distribution of pharmaceuticals in the environment; the pharmacokinetics of each drug; the structural transformation of the chemical compounds either through metabolism or natural degradation processes; and the potential bioaccumulation of the drugs.[20] More research is needed to determine the effects on humans of long-term exposure to low levels of PPCPs. The full effects of mixtures of low concentrations of different PPCPs is also unknown.[21]
Although research has shown that PPCPs are present in water bodies throughout the world, no studies have shown a direct effect on human health. However, the absence of empirical data cannot rule out the possibility of adverse outcomes due to interactions or long-term exposures to these substances. Because the amounts of these chemicals in the water supply may be in the parts per trillion or parts per billion, it is difficult to chemically determine the exact amounts present. Many studies[22] have therefore been focused to determining if the concentrations of these pharmaceuticals exist at or above the accepted daily intake (ADI) at which the designed biological outcomes can occur.[22]
In addition to the growing concerns about human health risks from pharmaceutical drugs via environmental exposures, many researchers have speculated about the potential for inducing an antibiotic resistance. One study found 10 different antibiotics in sewage treatment effluents, surface water, and sediments.[23] Some microbiologists believe that if antibiotic concentrations are higher than the minimum inhibitory concentrations (MICs) of a species of pathogenic bacteria, a selective pressure would be exerted and, as a result, antibiotic resistance would be selectively promoted. It has also been proven that at even sub-inhibitory concentrations (e.g., one-fourth of the MIC), several antibiotics are able to have an effect on gene expression (e.g., as shown for the modulation of expression of toxin-encoding genes in Staphylococcus aureus).[24] For reference the MIC of erythromycin that is effective against 90 percent of lab grown Campylobacter bacteria, the most common food-borne pathogen in the United States, is 60 ng/mL.[25] One study found that the average concentration of erythromycin, a commonly prescribed antibiotic, was 0.09 ng/mL in water treatment plant effluents,.[23] Additionally, transfer of genetic elements among bacteria has been observed under natural conditions in wastewater treatment plants, and selection of resistant bacteria has been documented in sewers receiving wastewaters from pharmaceutical plants.[24] Moreover, antibiotic resistant bacteria may also remain in sewage sludge and enter the food chain if the sludge is not incinerated but used as fertilizer on agricultural land.[19]
The relationship between risk perception and behavior is multifaceted. Risk management is most effective once the motivation behind the behavior of disposing unused pharmaceuticals is understood. There was little correlation found between the perception of risk and knowledge regarding pharmaceutical waste according to a study conducted by Cook and Bellis in 2001.[26] This study cautioned against the effectiveness of attempting to change the public’s behavior on these health issues by warning them of the risks associated with their actions. It is advised to take careful measures to inform the public in a way that does not impart guilt but rather public awareness. For example, a study carried out by Norlund and Garvill in Sweden (2003)[27] that found that some people may make a personal sacrifice in terms of comfort because they feel that it would be helpful to reduce further environmental damage caused by the use of cars. Awareness of air pollution problems was a factor in their decision to take action on a more environmentally favorable choice of transportation. Thus, the goal of Bound’s project encapsulates whether the perception of risk associated with pharmaceuticals has an effect on the way in which medication is commonly disposed.
In order to conduct this study, the pharmaceuticals were grouped by their therapeutic action in order to help participants identify them. The eight therapeutic groups are listed below: antibacterials, antidepressants, antihistamines, antiepileptics, hormone treatments, and lipid regulators. Next, a survey was created to examine the disposal patterns of the participants and their perception of the existing risk or threat against the environment. Respondents were asked the following questions in part one of the survey: 1. When and how they disposed of pharmaceuticals. 2. How they perceive the risk to the environment posed by pharmaceuticals. 3. To differentiate between the risks associated with different classed of pharmaceuticals. Part two of the survey involved each of the eight pharmaceutical groups described above individually. Finally, the third part asked information about the age, sex, profession, postcode, and education of participants. The sample size of participants was precise in comparison to the actual distribution of males and females in the UK: Sample- 54.8 percent were female and 45.2 percent male vs. Actual- the UK of 51.3 percent female to 48.7 percent male. Results showed that when a medication must be discarded, 63.2 percent of participants throw them in a bin, 21.8 percent return them to a pharmacist, and 11.5 percent dispose of them via the toilet/sink, while the remaining 3.5 percent keep them. Only half of the respondents felt like pharmaceuticals could potentially be harmful to the environment. Upon examination of factors relevant to risk perception, there was no definite link found between perception and education or income.
Dr. Bound noted that participation in altruistic activities such as Environmental Conservation groups may provide members with the ability to better grasp the effects of their actions in the environment. In regards to the aquatic environment, it is hard for one to perceive the favorable effects of properly disposing medication. There also exists the plausibility that a person’s behavior will only be affected if there is a severe risk to themselves or humans as opposed to an environmental threat. Even though there are serious threats of pharmaceutical pollution resulting in the feminization of certain fish, they have a lower priority because they are not easily understood or experienced by the general public. In Jonathan P. Bound’s opinion, the provision of information about exactly how to go about disposing unused medication properly in conjunction with risk education may have a more positive and forceful effect.
Environmental
While the full effects of most PPCPs on the environment are not understood, there is concern about the potential they have for harm because they may act unpredictably when mixed with other chemicals from the environment or concentrate in the food chain. Additionally, some PPCPS are active at very low concentrations, and are often released continuously in large or widespread quantities.
Because of the high solubility of most PPCPs, aquatic organisms are especially vulnerable to their effects. Researchers have found that a class of antidepressants may be found in frogs and can significantly slow their development. The increased presence of estrogen and other synthetic hormones in waste water due to birth control and hormonal therapies has been linked to increased feminization of exposed fish and other aquatic organisms.[28] The chemicals within these PPCP products could either affect the feminization or masculinization of different fishes, therefore affecting their reproductive rates.[29] In addition to being found only in waterways, the ingredients of some PPCPs can also be found in the soil. Since some of these substances take a long time or cannot be degraded biologically, they make their way up the food chain. Information pertaining to the transport and fate of these hormones and their metabolites in dairy waste disposal is still being investigated, yet research suggest that the land application of solid wastes is likely linked with more hormone contamination problems.[30] Not only does the pollution from PPCPs affect marine ecosystems, but also those habitats that depend on this polluted water.
There are various concerns about the effects of pharmaceuticals found in surface waters and specifically the threats against rainbow trout exposed to treated sewage effluents. Analysis of these pharmaceuticals in the blood plasma of fish compared to human therapeutic plasma levels have yielded vital information providing a means of assessing risk associated with medication waste in water. In a study by Dr. Jerker Fick from Umeå University[31] rainbow trout were exposed to undiluted, treated sewage water at three different sits in Sweden. They were exposed for a total of 14 days while 25 pharmaceuticals were measured in the blood plasma at different levels for analysis. The progestin Levonorgestrel was detected in fish blood plasma at concentrations between 8.5 and 12 ng mL-1 which exceed the human therapeutic plasma level. Studies show that the measured effluent level of Levonorgestrel in the three areas was shown to reduce the fertility of the rainbow trout.
The three sites chosen for field exposures were in located in Stockholm, Gothenburg, and Umeå. They were chosen according to their varying degrees of treatment technologies, geographic locations, and size. The effluent treatment includes active sludge treatment, nitrogen and phosphorus removal (except in Umeå), primary clarification, and secondary clarification. Juvenile rainbow trout were procured from Antens fiskodling AB, Sweden and Umlax AB, Sweden. The fish were exposed to aerated, undiluted, treated effluent. Since all of the sites underwent sludge treatment, it can be inferred that they are not representative of the low end of treatment efficacy. Of the 21 pharmaceuticals that were detected in the water samples, 18 were identified in the effluent, 17 in the plasma portion, and 14 pharmaceuticals were found in both effluent and plasma.
Current research
Starting in the mid-1960s, ecologists and toxicologists began to express concern about the potential adverse effects of pharmaceuticals in the water supply, but it wasn’t until a decade later that the presence of pharmaceuticals in water was well documented. Studies in 1975 and 1977 found clofibric and salicylic acids at trace concentrations in treated water.[32] Widespread concern about and research into the effect of PPCPs largely started in the early 1990s. Until this time, PPCPs were largely ignored because of their relative solubility and containment in waterways compared to more familiar pollutants like agrochemicals, industrial chemicals, and industrial waste and byproducts.[33] Since then, a great deal of attention has been directed to the ecological and physiological risk associated with pharmaceutical compounds and their metabolites in water and the environment. In the last decade, most research in this area has focused on steroid hormones and antibiotics. There is concern that steroid hormones may act as endocrine disruptors. Some research suggests that concentrations of ethinylestradiol, an estrogen used in oral contraceptive medications and one of the most commonly prescribed pharmaceuticals, can cause endocrine disruption in aquatic and amphibian wildlife in concentrations as low as 1 ng/L.[22]
Current research on PPCPs aims to answer these questions:[34]
- What is the effect of exposure to low levels of PPCPs over time?
- What is the effect of exposure to mixtures of chemicals?
- Are the effects acute (short-term) or chronic (long-term)?
- Are certain populations, such as the elderly, very young, or immuno-compromised, more vulnerable to the effects of these compounds?
- What is the effect of PPCPs on bacterial, fungal, and aquatic life?
- Are the levels of antibiotics in the aquatic environment sufficient to promote antibiotic resistance?
- What is the effect of exposure to steroid hormones on animal and human populations?
Pharmacoenvironmentology
Pharmacoenvironmentology is a branch of pharmacology and a form of pharmacovigilance which deals with entry of chemicals or drugs into the environment after elimination from humans and animals post-therapy. It deals specifically with those pharmacological agents that affect the environment via elimination through living organisms subsequent to pharmacotherapy.[35][36] It deals specifically with those pharmacological agents that have impact on the environment via elimination through living organisms subsequent to pharmacotherapy.[37][38][39]
Ecopharmacovigilance
Ecopharmacovigilance is the science and activities associated with the detection, evaluation, understanding and prevention of adverse effects of pharmaceuticals in the environment. This is close to the WHO definition of pharmacovigilance, the science aiming to capture any adverse effects of pharmaceuticals in humans after use.[40]
Ecopharmacology
Ecopharmacology concerns the entry of chemicals or drugs into the environment through any route and at any concentration disturbing the balance of ecology (ecosystem), as a consequence. Ecopharmacology is a broad term that includes studies of “PPCPs” irrespective of doses and route of entry into environment.[39][41][42]
Routes into the environment
Pharmaceutical residues may reach the environment by a number of different routes. It is generally assumed (albeit hardly verified) that the production of pharmaceuticals in industrialised countries is well controlled and unharmful to the environment, due to the local legal restrictions usually required to permit production. However, a substantial fraction of the global production of pharmaceuticals takes place in low-cost production countries like India and China. Recent reports from India demonstrate that such production sites may emit very large quantities of e.g. antibiotics, yielding levels of the drugs in local surface waters higher than those found in the blood of patients under treatment. The major route for pharmaceutical residues to reach the aquatic environment is most probably by excretion from patients undergoing pharma treatment. Since many pharmaceutical substances are not metabolized in the body they may be excreted in biologically active form, usually via the urine. Furthermore, many pharmaceutical substances are not fully taken up from the intestine (following oral administration in patients) into their blood stream. The fraction not taken up into the blood stream will remain in the gut and eventually be excreted via the faeces. Hence, both urine and faeces from treated patients contain pharmaceutical residues. Between 30 and 90% of the orally administered dose is generally excreted as active substance in the urine.[5] An additional source to environmental pollution with pharmaceuticals is improper disposal of unused or expired drug residues. In European countries take-back systems for such residues are usually in place (although not always utilized to full extent) while in e.g. the US only voluntary initiatives on a local basis exist. Though most of the waste goes to incineration and people are asked to throw unused or expired pharmaceuticals into the household waste investigations in Germany showed that up to 24% of liquid pharmaceuticals and 7% of tablets or ointments are disposed always or at least “rarely” via the toilet or sink.[19] Proper destruction of pharma residues should yield rest products without any pharmaceutical or ecotoxic activity. Furthermore, the residues should not act as components in the environmental formation of new such products. Incineration at a high temperature (>1000 degrees Celsius) is considered to fulfil the requirements, but even following such incineration residual ashes from the incineration should be properly taken care of.
Pharmaceuticals used in veterinary medicine, or as additives to animal food, pose a different problem, since they are excreted into soil or possibly open surface waters. It is well known that such excretions may affect terrestrial organisms directly, leading to extinction of exposed species (e.g. dung-beetles). Lipid-soluble pharma residues from veterinary use may bind strongly to soil particles, with little tendency to leak out to ground water or to local surface waters. More water-soluble residues may be washed out with rain or melting snow and reach both ground water and surface water streams.
Fate of pharmaceuticals in sewage treatment plants
Sewage treatment plants work with physical, chemical, and biological processes to remove nutrients and contaminants from waste water. Usually the sewage treatment plant (STP) is equipped with an initial mechanical separation of solid particles (cotton buds, cloth, hygien articles etc.) appearing in the incoming water. Following this there may be filters separating finer particles either occurring in the incoming water or developing as a consequence of chemical treatment of the water with flocculating agents. Many STPs also include one or several steps of biological treatment. By stimulating the activity of various strains of microorganisms physically their activity may be promoted to degrade the organic content of the sewage by up to 90% or more. In certain cases more advanced techniques are used as well. The today most commonly used advanced treatment steps especially in terms of micropollutants are
- membranes (which may be used instead of the biological treatment),
- ozonation,
- activated carbon (powdered or granulated),
- UV treatment,
- treatment with ferrate and
- sandfiltration (which is sometimes added as a last step after the aforementioned).
Several research projects are running to optimize the use of advanced sewage treatment techniques under different conditions. The advanced techniques will increase the costs for the sewage treatment substantially. In a European cooperation project between 2008 and 2012 in comparison 4 hospital waste water treatment facilities were developed in Switzerland, Germany, The Netherlands and Luxembourg to investigate the elimination rates of concentrated waste water with pharmaceutical “cocktails” by using different and combined advanced treatment technologies.[43] Especially the German STP at Marienhospital Gelsenkirchen showed the effects of a combination of membranes, ozone, powdered activated carbon and sand filtration.[44] But even a maximum of installed technologies could not eliminate 100% of all substances and especially radiocontrast agents are nearly impossible to eliminate. The investigations showed that depending on the installed technologies the treatment costs for such a hospital treatment facility may be up to 5.50 € per m2.[45] Other studies and comparisons expect the treatment costs to increase up to 10%, mainly due to energy demand.[46] It is therefore important to define best available technique before extensive infrastructure investments are introduced on a wide basis. The fate of incoming pharmaceutical residues in the STP is unpredictable. Some substances seem to be more or less completely eliminated, while others pass the different steps in the STP unaffected. There is no systematic knowledge at hand to predict how and why this happens.
Pharmaceutical residues that have been conjugated (bound to a bile acid) before being excreted from the patients may undergo de-conjugation in the STP, yielding higher levels of free pharmaceutical substance in the outlet from the STP than in its incoming water. Some pharmaceuticals with large sales volumes have not been detected in the incoming water to the STP, indicating that complete metabolism and degradation must have occurred already in the patient or during the transport of sewage from the household to the STP.
See also
- Drug pollution
- Plastic particle water pollution
- Environmental issue
- Water pollution
- Environmental persistent pharmaceutical pollutant
References
- 1 2 3 "Pharmaceuticals and Personal Care Products". Washington, D.C.: U.S. Environmental Protection Agency (EPA). 2012. Retrieved 2015-07-23.
- ↑ DIRECTIVE 2013/39/EU of 12 August 2013 amending Directives 2000/60/EC and 2008/105/EC as regards priority substances in the field of water policy
- 1 2 Doerr-MacEwen NA, Haight ME (November 2006). "Expert stakeholders' views on the management of human pharmaceuticals in the environment". Environ Manage. 38 (5): 853–66. doi:10.1007/s00267-005-0306-z. PMID 16955232.
- ↑ Donn J. (2009). Tons of Released Drugs Taint U.S. Water. AP.
- 1 2 BIO Intelligence Service (2013), Study on the environmental risks of medicinal products, Final Report prepared for Executive Agency for Health and Consumers
- ↑ "National Take Back Initiative". Office of Diversion Control, United States Drug Enforcement Administration. Retrieved 04/05/2011. Check date values in:
|access-date=(help) - ↑ "Long Battle for State Drug Take-Back Program Must Continue". The Olympian. 2011-03-13. Retrieved 04/05/2011. Check date values in:
|access-date=(help) - ↑ "Proper Disposal of Prescription Drugs" (PDF). U.S. Office of National Drug Policy. October 2009. Archived from the original (PDF) on 2010-03-31.
- ↑ Siegrist, H., Ternes, T.A., Joss, A., (2004) Scrutinizing Pharmaceuticals and Personal Care Products in Wastewater Treatment Journal of Environmental Science & Technology,38 392A-399A
- ↑ K. Kümmerer. Pharmaceuticals in the Environment – A Brief Summary. In: Kümmerer, Klaus (Ed.). Pharmaceuticals in the Environment (ISBN 978-3-540-74663-8), 3rd Edition: Springer Berlin Heidelberg, 2008: 3-21
- ↑ Bohannon, J. "Hard Data on Hard Drugs, Grabbed From the Environment." Science Magazine, published 6 April 2007. Accessed 20 April 2009.
- ↑ "Origins and Fate of PPCPs in the Environment" (PDF). Pharmaceuticals and Personal Care Products. EPA, National Exposure Research Laboratory. March 2006.
- ↑ Tong, A.Y.; Peake, B.; Braund, R. (2011). "Disposal practices for unused medications around the world.". Environment International. 37: 292–298. doi:10.1016/j.envint.2010.10.002.
- ↑ EU project report summary “Pharmaceutical Input and Elimination from Local Sources”, 2012
- ↑ Buxton, H.T.; Kolpin, D.W. (June 2002). "Pharmaceuticals, Hormones, and Other Organic Wastewater Contaminants in U.S. Streams". USGS Fact Sheet FS-027-02. Reston, VA: U.S. Geological Survey.
- ↑ "Pharmaceutical and Personal Care Products in Drinking Water Supplies." The Groundwater Foundation. Accessed 19 April 2009.
- ↑ Hernando, M.D.; Mezcua, M.; Fernandez-Alba, A.R.; Barcelo, D. (2006). "Environmental Risk Assessment of Pharmaceutical Residues in Wastewater Effluents, Surface Waters and Sediments". Talanta. 69: 334–342. doi:10.1016/j.talanta.2005.09.037.
- ↑ Jiang, Jheng-Jie; Lee, Chon-Lin; Fang, Meng-Der; et al. (15 December 2014). "Impacts of Emerging Contaminants on Surrounding Aquatic Environment from a Youth Festival". Environmental Science & Technology. 49: 792–799. doi:10.1021/es503944e. PMID 25495157. Lay summary – Pharmaceutical Processing (15 January 2015).

- 1 2 3 EU project noPILLS in waters, final report 2015
- ↑ Daughton, C.G. (2008). "Pharmaceuticals as Environmental Pollutants: the Ramifications for Human Exposure". International Encyclopedia of Public Health. 5: 66–122. doi:10.1016/b978-012373960-5.00403-2.
- ↑ "Pharmaceuticals and personal care products in drinking water." American Water Works Association. Accessed 20 April 2009.
- 1 2 3 Daughton, C.G. (2008). "Pharmaceuticals as Environmental Pollutants: the Ramifications for Human Exposure". International Encyclopedia of Public Health. 5: 66–102. doi:10.1016/b978-012373960-5.00403-2.
- 1 2 Hernando, M.D.; Mezcua, M.; Fernández-Alba, AR.; Barceló, D. (2006). "Environmental Risk Assessment of Pharmaceutical Residues in Wastewater Effluents, Surface Waters and Sediments". Talanta. 69: 334–342. doi:10.1016/j.talanta.2005.09.037.
- 1 2 Segura, P.A.; Francois, M.; Gagnon, C.; Sauve, S. (May 2005). "Review of the Occurrence of Anti-infectives in Contaminated Wastewaters and Natural and Drinking Waters". Environmental Health Perspectives. 117 (5): 675–684. doi:10.1289/ehp.11776.
- ↑ Modolo, J.R.; Giuffrida, R.; de M. Lopes, C.R. (July 2003). "ANTIMICROBIAL SUSCEPTIBILITY OF 51 CAMPYLOBACTER STRAINS ISOLATED FROM DIARRHEIC AND DIARRHEA-FREE DOGS". Arq. Inst. Biol. 70 (3): 283–286.
- ↑ Cook, Bellis (2001). "Knowing the risk: relationships between behaviour and health knowledge. Public Health 115, 54-61.". Public Health.
- ↑ Nordlund,, A.M. (2003). "Effects of values, problem awareness, and personal norm on willingness to reduce personal car use.". J. Environment Psychol.
- ↑ "Pharmaceuticals and Personal Care Products in the Environment." Washington State University. Accessed 20 April 2009.
- ↑ Siegrist, H., Ternes, T.A., Joss, A., (2004) Scrutinizing Pharmaceuticals and Personal Care Products in Wastewater Treatment Journal of Environmental Science & Technology,38 392A-399A
- ↑ Zheng, W., Yates,S.R., Bradford, S.A. ( 2007) Analysis of Steroid Hormones in a Typical Dairy Waste Disposal System Journal of Environmental Science & Technology, 42, 530-535
- ↑ Fick, Jerker (2009). "Therapeutic Levels of Levonorgestrel Detected in Blood Plasma of Fish: Results from Screening Rainbow Trout Exposed to Treated Sewagae Effluents". Evniron. Sci. Technol.
- ↑ Snyder, S.; Westerhoff, P.; Yoon, Y.; Sedlak, D. (2003). "Pharmaceuticals, Personal Care Products, and Endocrine Disruptors in Water: Implications for the Water Industry". Environmental Engineering Science. 20 (5).
- ↑ "Chemicals from Pharmaceuticals and Personal Care Products." Water Encyclopedia. Accessed 20 April 2009.
- ↑ "Pharmaceuticals and Personal Care Products: An Emerging Issue." The Groudwater Foundation. Accessed 20 April 2009.
- ↑ Rahman, SZ; Khan, RA (Dec 2006). "Environmental pharmacology: A new discipline". Indian J Pharmacol. 38 (4): 229–30. doi:10.4103/0253-7613.27017.
- ↑ Daughton, Christian G; Ruhoy, Ilene Sue. The Afterlife of Drugs and the Role of PharmEcovigilance. Drug Safety 2008; 31(12): 1069-1082
- ↑ Rahman, SZ; Khan RA; Gupta V; Misbah Uddin (2008). "Chapter 2: Pharmacoenvironmentology – Ahead of Pharmacovigilance". In Rahman SZ, Shahid M & Gupta A. An Introduction to Environmental Pharmacology (PDF) (1st ed.). Aligarh, India: Ibn Sina Academy of Medieval Medicine and Sciences. pp. 35–52. ISBN 978-81-906070-4-9.
- ↑ Rahman, SZ; Khan, RA; Gupta, V; Uddin, Misbah (July 2007). "Pharmacoenvironmentology – A Component of Pharmacovigilance". Environmental Health. 6 (20). doi:10.1186/1476-069X-6-20. PMC 1947975
 . PMID 17650313.
. PMID 17650313. - 1 2 Ilene Sue Ruhoy, Christian G. Daughton. Beyond the medicine cabinet: An analysis of where and why medications accumulate. Environment International 2008, Vol. 34 (8): 1157-1169
- ↑ Ecopharmacovigilance (AstraZeneca) Archived January 26, 2012, at the Wayback Machine.
- ↑ Hashemi, Zahra (2008). "Addendum: Terminologies related to Drug Safety". In Rahman SZ, Shahid M & Gupta A. An Introduction to Environmental Pharmacology (1st ed.). Aligarh: Ibn Sina Academy of Medieval Medicine and Sciences. pp. 257–259. ISBN 978-81-906070-4-9.
- ↑ Rahman, SZ; Khan, RA; Gupta, V; Uddin, Misbah (July 2007). "Pharmacoenvironmentology–A Component of Pharmacovigilance". Environmental Health. 6 (20): 20. doi:10.1186/1476-069X-6-20. PMC 1947975
 . PMID 17650313.
. PMID 17650313. - ↑ webpage EU Interreg IV B project Pharmaceutical Input and Elimination from Local Sources
- ↑ film portray of the hospital waste water treatment facility Marienhospital Gelsenkirchen
- ↑ EU project PILLS summary
- ↑ report of the German Federal Environment Agency
Further reading
- Kümmerer, K. (2010). "Pharmaceuticals in the Environment". Annual Review of Environment and Resources. 35: 57–75. doi:10.1146/annurev-environ-052809-161223.
- Kumar, R. R.; Lee, J. T.; Cho, J. Y. (2012). "Fate, occurrence, and toxicity of veterinary antibiotics in environment". Journal of the Korean Society for Applied Biological Chemistry. 55 (6): 701. doi:10.1007/s13765-012-2220-4.
External links
- "How to Dispose of Unused Medicines". Consumer Updates. U.S. Food and Drug Administration. 2015-06-04.