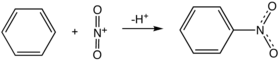Nitro compound

Nitro compounds are organic compounds that contain one or more nitro functional groups (−NO2). The nitro group is one of the most common explosophores (functional group that makes a compound explosive) used globally. The nitro group is also strongly electron-withdrawing. Because of this property, C−H bonds alpha (adjacent) to the nitro group can be acidic. For similar reasons, the presence of nitro groups in aromatic compounds retards electrophilic aromatic substitution but facilitates nucleophilic aromatic substitution. Nitro groups are rarely found in nature, being almost invariably produced by nitration reactions starting with nitric acid.
Production and occurrence
Preparation of aromatic nitro compounds
Aromatic nitro compounds are typically synthesized by nitration. Nitration is achieved using a mixture of nitric acid and sulfuric acid, which produce the nitronium ion (NO2+), which the electrophile:
The nitration product produced on the largest scale, by far, is nitrobenzene. Many explosives are produced by nitration including trinitrophenol (picric acid), trinitrotoluene (TNT), and trinitroresorcinol (styphnic acid).[1] Another but more specialized method for making aryl-NO2 group starts from halogenated phenols, is the Zinke nitration.
Preparation of aliphatic nitro compounds
Aliphatic nitro compounds can be synthesized by various methods; notable examples include:
- Free radical nitration of alkanes.[2] The reaction produces fragments from the parent alkane, creating a diverse mixture of products; for instance, nitromethane, nitroethane, 1-nitropropane, and 2-nitropropane are produced by treating propane with nitric acid in the gas phase (e.g. 350–450 °C and 8–12 atm).
- Nucleophilic substitution reactions between halocarbons[3] or organosulfates[4] with silver or alkali nitrite salts.
- Nitromethane can be produced in the laboratory by treating sodium chloroacetate with sodium nitrite.[5]
- Oxidation of oximes[6] or primary amines.[7]
Tar Meer Reaction
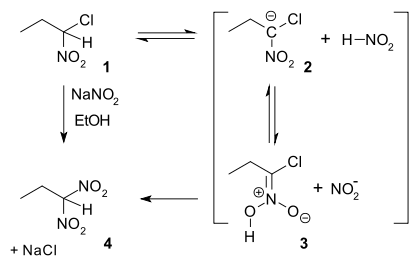
In nucleophilic aliphatic substitution, sodium nitrite (NaNO2) replaces an alkyl halide. In the so-called Ter Meer reaction (1876) named after Edmund ter Meer,[8] the reactant is a 1,1-halonitroalkane:
The reaction mechanism is proposed in which in the first slow step a proton is abstracted from nitroalkane 1 to a carbanion 2 followed by protonation to a nitronate 3 and finally nucleophilic displacement of chlorine based on an experimentally observed hydrogen kinetic isotope effect of 3.3.[9] When the same reactant is reacted with potassium hydroxide the reaction product is the 1,2-dinitro dimer[10]
Occurrence in nature
Chloramphenicol is a rare example of a naturally-occurring nitro compound. At least some naturally occurring nitro groups arised by the oxidation of amino groups.[11] 2-Nitrophenol is an aggregation pheromone of ticks.
Examples of nitro compounds are rare in nature. 3-Nitropropionic acid found in fungi and plants (Indigofera). Nitropentadecene is a defense compound found in termites. Nitrophenylethane is found in Aniba canelilla.[12] Nitrophenylethane is also found in members of the Annonaceae, Lauraceae and Papaveraceae.[13]
Reactions of aliphatic nitro compounds
Reduction
Nitro compounds participate in several organic reactions, the most important being their reduction to the corresponding amines:
- RNO2 + 3 H2 → RNH2 + 2 H2O
Acid-base reactions
Nitroalkanes are somewhat acidic. The pKas of nitromethane and isopropyl nitrate, are 17.2 and 16.9 in DMSO solution. These values suggest aqueous pKas of around 11.[14] In other words, these carbon acids can be deprotonated in aqueous solution. The conjugate base is called nitronate. Nitronates protonate at oxygen to give a tautomer of nitroalkyl precursor. This process is the start of a reaction that converts nitronates to aldehydes or ketones, called the Nef reaction.
Condensation reactions
Nitromethane undergoes base-catalyzed additions to aldehydes in 1,2-addition in the nitroaldol reaction. Similarly, it adds to alpha-beta unsaturated carbonyl compounds as a 1,4-addition in the Michael reaction as a Michael donor. Nitroalkenes are Michael acceptors in the Michael reaction with enolate compounds.[15][16]
Biochemical reactions
Many flavin-dependent enzymes are capable of oxidizing aliphatic nitro compounds to less-toxic aldehydes and ketones. Nitroalkane oxidase and 3-nitropropionate oxidase oxidize aliphatic nitro compounds exclusively, whereas other enzymes such as glucose oxidase have other physiological substrates.[17]
Reactions of aromatic nitro compounds
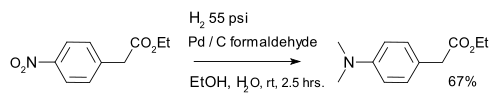
Reduction of aromatic nitro compounds with hydrogen over a meta catalysts gives anilines. Virtually all aromatic amines (anilines) are derived from nitroaromatics. A variation is formation of a dimethylaminoarene with palladium on carbon and formaldehyde:[18]
The Leimgruber–Batcho, Bartoli and Baeyer–Emmerling indole syntheses begin with aromatic nitro compounds. Indigo can be synthesized in a condensation reaction from ortho-nitrobenzaldehyde and acetone in strongly basic conditions in a reaction known as the Baeyer–Drewson indigo synthesis.
Explosions
Explosive decomposition of organo nitro compounds are redox reactions, wherein both the oxidant (nitro group) and the fuel (hydrocarbon substituent) are bound within the same molecule. The explosion process generates heat by forming highly stable products including molecular nitrogen (N2), carbon dioxide, and water. The explosive power of this redox reaction is enhanced because these stable products are gases at mild temperatures. Many contact explosives contain the nitro group.
See also
- Functional group
- Reduction of nitro compounds
- Nitration
- Nitrite (also an NO2 group, but bonds differently)
- Nitroalkene
- Nitroglycerin
References
- ↑ Gerald Booth "Nitro Compounds, Aromatic" 'Ullmann's Encyclopedia of Industrial Chemistry', 2005, Wiley-VCH, Weinheim. doi:10.1002/14356007.a17_411
- ↑ Markofsky, Sheldon; Grace, W.G. (2000). "Nitro Compounds, Aliphatic". Ullmann's Encyclopedia of Industrial Chemistry. doi:10.1002/14356007.a17_401.
- ↑ Kornblum, N.; Ungnade, H. E. (1963). "1-Nitroöctane". Organic Syntheses. 4: 724. doi:10.15227/orgsyn.038.0075.
- ↑ Walden, P. (1907). "Zur Darstellung aliphatischer Sulfocyanide, Cyanide und Nitrokörper". Berichte der deutschen chemischen Gesellschaft. 40 (3): 3214–3217. doi:10.1002/cber.19070400383.
- ↑ Whitmore, F. C.; Whitmore, Marion G. (1923). "Nitromethane". Organic Syntheses. 1: 401. doi:10.15227/orgsyn.003.0083.
- ↑ Olah, George A.; Ramaiah, Pichika; Chang-Soo, Lee; Prakash, Surya (1992). "Convenient Oxidation of Oximes to Nitro Compounds with Sodium Perborate in Glacial Acetic Acid". Synlett. 4: 337–339. doi:10.1055/s-1992-22006.
- ↑ Ehud, Keinan; Yehuda, Mazur (1977). "Dry ozonation of amines. Conversion of primary amines to nitro compounds". The Journal of Organic Chemistry. 42 (5): 844–847. doi:10.1021/jo00425a017.
- ↑ Edmund ter Meer (1876). "Ueber Dinitroverbindungen der Fettreihe". Justus Liebigs Annalen der Chemie. 181 (1): 1–22. doi:10.1002/jlac.18761810102.
- ↑ aci-Nitroalkanes. I. The Mechanism of the ter Meer Reaction M. Frederick Hawthorne J. Am. Chem. Soc.; 1956; 78(19) pp 4980–4984; doi:10.1021/ja01600a048
- ↑ 3-Hexene, 3,4-dinitro- D. E. Bisgrove, J. F. Brown, Jr., and L. B. Clapp. Organic Syntheses, Coll. Vol. 4, p.372 (1963); Vol. 37, p.23 (1957). (Article)
- ↑ Georg Zocher, Robert Winkler, Christian Hertweck, Georg E. Schulz "Structure and Action of the N-oxygenase AurF from Streptomyces thioluteus" J. Molecular Biology (2007) 373, 65–74. doi:10.1016/j.jmb.2007.06.014
- ↑ José Guilherme S. Maia, Eloísa Helena A. Andrade "Database of the Amazon aromatic plants and their essential oils" Quim. Nova, (2009) 32(3), 595–622, 2009
- ↑ Klaus Kubitzki, Jens G. Rohwer, Volker Bittrich "Flowering Plants · Dicotyledons: Magnoliid, Hamamelid and Caryophyllid Families" 1993, Springer-Verlag, Berlin
- ↑ Bordwell, F. G.; Satish, A. V., "Is Resonance Important in Determining the Acidities of Weak Acids or the Homolytic Bond Dissociation Enthalpies (BDEs) of Their Acidic H-A Bonds?", J. Am. Chem. Soc. 1994, volume 116, 8885-8889. doi:10.1021/ja00099a004
- ↑ Ranganathan, Darshan; Rao, Bhushan; Ranganathan, Subramania; Mehrotra, Ashok & Iyengar, Radha (1980). "Nitroethylene: a stable, clean, and reactive agent for organic synthesis". The Journal of Organic Chemistry. 45 (7): 1185–1189. doi:10.1021/jo01295a003. Retrieved 5 January 2014.
- ↑ Jubert, Carole & Knochel, Paul (1992). "Preparation of polyfunctional nitro olefins and nitroalkanes using the copper-zinc reagents RCu(CN)ZnI". The Journal of Organic Chemistry. 57 (20): 5431–5438. doi:10.1021/jo00046a027. Retrieved 5 January 2014.
- ↑ Nagpal, Akanksha; Valley, Michael P.; Fitzpatrick, Paul F.; Orville, Allen M. (2006). "Crystal Structures of Nitroalkane Oxidase: Insights into the Reaction Mechanism from a Covalent Complex of the Flavoenzyme Trapped during Turnover". Biochemistry. 45 (4): 1138–50. doi:10.1021/bi051966w. PMC 1855086
 . PMID 16430210.
. PMID 16430210. - ↑ Organic Syntheses, Coll. Vol. 5, p.552 (1973); Vol. 47, p.69 (1967). http://orgsynth.org/orgsyn/pdfs/CV5P0552.pdf
| Wikimedia Commons has media related to Nitro compounds. |