Prader–Willi syndrome
| Prader-Willi syndrome | |
|---|---|
| Synonyms | Labhart-Willi syndrome, Prader's syndrome, Prader-Labhart-Willi-Fanconi syndrome[1] |
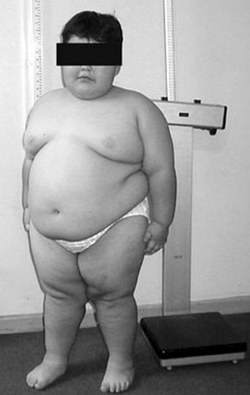 | |
| Eight year old with Prader–Willi syndrome,[2] exhibiting characteristic obesity | |
| Pronunciation | /ˈprɑːdər ˈvɪli/ |
| Classification and external resources | |
| Specialty | medical genetics, pediatrics |
| ICD-10 | Q87.1 |
| ICD-9-CM | 759.81 |
| OMIM | 176270 |
| DiseasesDB | 10481 |
| MedlinePlus | 001605 |
| eMedicine | ped/1880 |
| Patient UK | Prader–Willi syndrome |
| MeSH | D011218 |
| GeneReviews | |
| Orphanet | 739 |
Prader–Willi syndrome (PWS) is a genetic disorder due to loss of function of specific genes on chromosome 15.[3] In newborns symptoms include weak muscles, poor feeding, and slow development. In childhood the person becomes constantly hungry which often leads to obesity and type 2 diabetes. There is also typically mild to moderate intellectual impairment and behavioral problems. Often the forehead is narrow, hands and feet small, height short, skin light in color, and they are unable to have children.[3]
About 70% of cases occur when part of the father's chromosome 15 is deleted. In another 25% of cases the person has two copies of chromosome 15 from their mother and none from their father. As parts of the chromosome from the mother are turned off they end up with no working copies of certain genes. PWS is not generally inherited but instead the genetic changes happen during the formation of the egg, sperm, or in early development.[3] There are no known risk factors. Those who have one child with PWS have less than a 1% chance of the next child being affected.[4] A similar mechanism occurs in Angelman syndrome except there is a defective chromosome 15 from the mother or two copies from the father.[5][6]
Prader–Willi syndrome has no cure. Treatment, however, may improve outcomes, especially if carried out early.[7] In newborns feeding difficulties may be supported with feeding tubes. Strict food supervision is typically required starting around the age of three in combination with an exercise program. Growth hormone therapy also improves outcomes. Counseling and medications may help with some behavioral problems. Group homes are often necessary in adulthood.[8]
PWS affects between 1 in 10,000 and 1 in 30,000 people.[3] Males and females are affected equally.[4] The condition is named after Andrea Prader, Heinrich Willi, and Alexis Labhart who described it in detail in 1956.[1] An earlier description occurred in 1887 by John Langdon Down.[9][10]
Signs and symptoms

There are many signs and symptoms of Prader–Willi syndrome. The symptoms can range from poor muscle tone during infancy to behavioral problems in early childhood. Some symptoms that are usually found in infants, besides poor muscle tone, would be a lack of eye coordination; some are born with almond-shaped eyes; and due to poor muscle tone the infant may not have a strong sucking reflex. Their cry is weak, and they have difficulty waking up. Another sign of this condition is a thin upper lip.
More aspects seen in a clinical overview include hypotonia and abnormal neurologic function, hypogonadism, developmental and cognitive delays, hyperphagia and obesity, short stature, and behavioral and psychiatric disturbances.[11]
Holm et al. (1993) describe the following features and signs as pretest indicators of PWS, although not all will be present.
Uterus and birth
- Reduced fetal movement
- Frequent abnormal fetal position
- Occasional polyhydramnios (excessive amniotic fluid)
- Often breech or caesarean births
- Lethargy
- Hypotonia
- Feeding difficulties (due to poor muscle tone affecting sucking reflex)
- Difficulties establishing respiration
- Hypogonadism
Childhood
- Delayed milestones/intellectual delay
- Excessive sleeping
- Strabismus ('crossed eyes')
- Scoliosis (often not detected at birth)
- Cryptorchidism
- Speech delay
- Poor physical coordination
- Hyperphagia (over-eating) begins between the age of 2 and 8, and continues on throughout adulthood. Noted change from feeding difficulties in infancy.
- Excessive weight gain
- Sleep disorders
- Delayed puberty
- Short stature
- Obesity
- Extreme flexibility
Adulthood
- Infertility (males and females)
- Hypogonadism
- Sparse pubic hair
- Obesity
- Hypotonia (low muscle tone)
- Learning disabilities/borderline intellectual functioning (but some cases of average intelligence)
- Prone to diabetes mellitus
- Extreme flexibility
Physical appearance
- Prominent nasal bridge
- Small hands and feet with tapering of fingers
- Soft skin, which is easily bruised
- Excess fat, especially in the central portion of the body
- High, narrow forehead
- Thin upper lip
- Downturned mouth
- Almond-shaped eyes
- Light skin and hair relative to other family members
- Lack of complete sexual development
- Frequent skin picking
- Striae
- Delayed motor development
Neuro-cognitive
Individuals with PWS are at risk of learning and attention difficulties. Curfs and Fryns (1992) conducted research into the varying degrees of learning disability found in PWS.[12] Their results, using a measure of IQ, were as follows:
- 5%: IQ above 85 (high to low average intelligence)
- 27%: IQ 70–85 (borderline intellectual functioning)
- 39%: IQ 50–70 (mild intellectual disability)
- 27%: IQ 35–50 (moderate intellectual disability)
- 1%: IQ 20–35 (severe intellectual disability)
- <1%: IQ <20 (profound intellectual disability)
Cassidy found that 40% of individuals with PWS have borderline/low average intelligence,[13] a figure higher than the 32% found in Curfs and Fryns' study.[12] However, both studies suggest that most individuals (50–65%) fall within the mild/borderline/low average intelligence range.
Parents report that some children have IQs >110 and function normally in school.
Children with PWS show an unusual cognitive profile. They are often strong in visual organization and perception, including reading and vocabulary, but their spoken language (sometimes affected by hypernasality) is generally poorer than their comprehension. A marked skill in completing jigsaw puzzles has been noted,[14][15] but this may be an effect of increased practice.[16]
Auditory information processing and sequential processing are relatively poor, as are arithmetic and writing skills, visual and auditory short-term memory and auditory attention span. These sometimes improve with age, but deficits in these areas remain throughout adulthood.[14]
Behavioral
Prader–Willi syndrome is frequently associated with a constant, extreme, ravenous insatiable appetite which persists no matter how much the patient eats, often resulting in morbid obesity. Caregivers need to strictly limit the patients' access to food, usually by installing locks on refrigerators and on all closets and cabinets where food is stored.[17] It is the most common genetic cause of morbid obesity in children.[18] There is currently no consensus as to the cause for this symptom, although genetic abnormalities in chromosome 15 disrupt the normal functioning of the hypothalamus.[13] Given that the hypothalamus arcuate nucleus regulates many basic processes, including appetite, there may well be a link. In the hypothalamus of people with PWS, nerve cells that produce oxytocin, a hormone thought to contribute to satiety, have been found to be abnormal.
People with Prader–Willi syndrome have high ghrelin levels, which are thought to directly contribute to the increased appetite, hyperphagia, and obesity seen in this syndrome.[19] Cassidy states the need for a clear delineation of behavioral expectations, the reinforcement of behavioural limits and the establishment of regular routines.
The main mental health difficulties experienced by people with PWS include compulsive behaviour (usually manifested in skin picking) and anxiety.[14][20] Psychiatric symptoms, for example, hallucinations, paranoia and depression, have been described in some cases[14] and affect approximately 5–10% of young adults.[13] Patients are also often extremely stubborn and prone to anger.[17] Psychiatric and behavioural problems are the most common cause of hospitalization.[21]
It is typical for to 70–90% of affected individuals to develop behavioral patterns in early childhood.[11] Aspects of these patterns can include stubbornness, temper tantrums, controlling and manipulative behavior, difficulty with change in routine, and compulsive-like behaviors.[11]
Endocrine
There are several aspects of PWS that support the concept of growth hormone deficiency in individuals with PWS. Specifically, individuals with PWS have short stature, are obese with abnormal body composition, have reduced fat free mass (FFM), have reduced lean body mass (LBM) and total energy expenditure, and have decreased bone density.
PWS is characterized by hypogonadism. This is manifested as undescended testes in males and benign premature adrenarche in females. Testes may descend with time or can be managed with surgery or testosterone replacement. Adrenarche may be treated with hormone replacement therapy.
Ophthalmologic
PWS is commonly associated with development of strabismus. In one study,[22] over 50% of patients had strabismus, mainly esotropia.
Genetics
PWS is an autosomal dominant disorder caused by the deletion of the paternal copies of the SNRPN and necdin genes along with clusters of snoRNAs: SNORD64, SNORD107, SNORD108 and two copies of SNORD109, 29 copies of SNORD116 (HBII-85) and 48 copies of SNORD115 (HBII-52). These are on chromosome 15 located in the region 15q11-13.[23][24][25][26] This so-called PWS/AS region may be lost by one of several genetic mechanisms which, in the majority of instances occurs through chance mutation. Other less common mechanisms include; uniparental disomy, sporadic mutations, chromosome translocations, and gene deletions. Due to imprinting, the maternally inherited copies of these genes are virtually silent, only the paternal copies of the genes are expressed.[27][28] PWS results from the loss of paternal copies of this region. Deletion of the same region on the maternal chromosome causes Angelman syndrome (AS). PWS and AS represent the first reported instances of imprinting disorders in humans.
The risk to the sibling of an affected child of having PWS depends upon the genetic mechanism which caused the disorder. The risk to siblings is <1% if the affected child has a gene deletion or uniparental disomy, up to 50% if the affected child has a mutation of the imprinting control region, and up to 25% if a parental chromosomal translocation is present. Prenatal testing is possible for any of the known genetic mechanisms.
A microdeletion in one family of the snoRNA HBII-52 has excluded it from playing a major role in the disease.[29]
Studies of human and mouse model systems have shown deletion of the 29 copies of the C/D box snoRNA SNORD116 (HBII-85) to be the primary cause of Prader–Willi syndrome.[30][31][32][33][34]
Diagnosis
PWS affects approximately 1 in 10,000 to 1 in 25,000 newborns.[35] There are more than 400,000 people who live with PWS around the world.[36] It is traditionally characterized by hypotonia, short stature, hyperphagia, obesity, behavioral issues (specifically OCD-like behaviors), small hands and feet, hypogonadism, and mild intellectual disability.[35] However, with early diagnosis and early treatment (such as with growth hormone therapy), the prognosis for persons with PWS is beginning to change. Like autism, PWS is a spectrum disorder and symptoms can range from mild to severe and may change throughout the person's lifetime. Various organ systems are affected.
Traditionally, Prader–Willi syndrome was diagnosed by clinical presentation. Currently, the syndrome is diagnosed through genetic testing; testing is recommended for newborns with pronounced hypotonia. Early diagnosis of PWS allows for early intervention as well as the early prescription of growth hormone. Daily recombinant growth hormone (GH) injections are indicated for children with PWS. GH supports linear growth and increased muscle mass, and may lessen food preoccupation and weight gain.
The mainstay of diagnosis is genetic testing, specifically DNA-based methylation testing to detect the absence of the paternally contributed Prader–Willi syndrome/Angelman syndrome (PWS/AS) region on chromosome 15q11-q13. Such testing detects over 97% of cases. Methylation-specific testing is important to confirm the diagnosis of PWS in all individuals, but especially those who are too young to manifest sufficient features to make the diagnosis on clinical grounds or in those individuals who have atypical findings.
Prader–Willi syndrome is often misdiagnosed as other syndromes due to many in the medical community's unfamiliarity with PWS.[18] Sometimes it is misdiagnosed as Down syndrome, simply because of the relative frequency of Down syndrome compared to PWS.[18]
Treatment
Prader–Willi syndrome has no cure; however, several treatments are in place to lessen the condition's symptoms. During infancy, subjects should undergo therapies to improve muscle strength. Speech and occupational therapy are also indicated. During the school years, children benefit from a highly structured learning environment as well as extra help. The largest problem associated with the syndrome is severe obesity. Access to food must be strictly supervised and limited, usually by installing locks on all food-storage places including refrigerators.[17]
Because hypotonia can be a symptom of PWS, it is vital to provide proper nutrition during infancy. It is also very important to stress physical activity in individuals with PWS for all ages in order to optimize strength and promote a healthy lifestyle.[11]
Prescription of daily recombinant growth hormone injections are indicated for children with PWS. GH supports linear growth and increased muscle mass, and may lessen food preoccupation and weight gain.[37][38][39]
Because of severe obesity, obstructive sleep apnea is a common sequela, and a positive airway pressure machine is often needed. There may come a time when a person who has been diagnosed with PWS may have to undergo surgical procedures. One surgery that has proven to be unsuccessful for treating the obesity is gastric bypass. Patients with Prader–Willi syndrome have a very high tolerance to pain; therefore they may be experiencing significant abdominal symptoms such as acute gastritis, appendicitis, or cholecystitis and not be aware of it until later.
Zafgen, Inc. is currently conducting Phase 3 trials of beloranib relating to controlling the weight and appetite of Prader–Willi syndrome patients,[40][41][42] with 2a results showing 8.1% reduced body fat after 4 weeks (at the highest study dose of 1.8 mg) and decreased appetite, despite a 50% increase in daily calorie allowance.[43][44] In December, 2015 Zafgen halted a Phase III clinical trial for Prader–Willi syndrome after second patient death in order to determine whether the deaths were treatment related.[45]
Behavior and psychiatric problems should be detected early for the best results. These issues are best when treated with parental education and training. Sometimes medication is introduced as well. Serotonin agonists have been most effective in lessening temper tantrums and improving compulsivity.[11]
Society and culture
%2C_de_Juan_Carre%C3%B1o_de_Miranda..jpg)
Despite its rarity, Prader–Willi syndrome has been often referenced in popular culture, partly due to the fascination surrounding the insatiable appetite and obesity that are symptoms.
Prader–Willi syndrome has been depicted and documented several times in television. A fictional individual with Prader–Willi syndrome featured in the episode "Dog Eat Dog" of the television series CSI: Crime Scene Investigation, which aired on November 24, 2005.[47] In the UK media in July 2007, Channel 4 aired a 2006 documentary called Can't Stop Eating, surrounding the everyday lives of two people with Prader–Willi syndrome, Joe and Tamara.[48] In the May 9, 2010 episode of Extreme Makeover: Home Edition, Sheryl Crow helped Ty Pennington rebuild a home for a family whose youngest son, Ethan Starkweather, was suffering from Prader–Willi syndrome.[49] In the March 22, 2012 episode of Mystery Diagnosis on the Discovery Health channel, Conor Heybach, who has Prader–Willi syndrome, shared his story of how he was diagnosed with it.[50]
In December 2011 the Taipei Times, in Taiwan, highlighted the tragedy of a taxi driver who had killed himself and his nine-year-old daughter who had the condition, in what police described as a "probable murder-suicide."[51]
See also
References
- 1 2 "Prader-Labhardt-Willi syndrome". Whonamedit?. Retrieved 20 August 2016.
- ↑ Cortés M, F; Alliende R, MA; Barrios R, A; Curotto L, B; Santa María V, L; Barraza O, X; Troncoso A, L; Mellado S, C; Pardo V, R (January 2005). "[Clinical, genetic and molecular features in 45 patients with Prader-Willi syndrome].". Revista medica de Chile. 133 (1): 33–41. PMID 15768148.
- 1 2 3 4 "Prader-Willi syndrome". Genetics Home Reference. June 2014. Retrieved 19 August 2016.
- 1 2 "How many people are affected/at risk for Prader-Willi syndrome (PWS)?". NICHD. 2014-01-14. Retrieved 20 August 2016.
- ↑ "Prader-Willi Syndrome (PWS): Other FAQs". NICHD. 2014-01-14. Retrieved 2016-08-19.
- ↑ "Angelman syndrome". Genetic Home Reference. May 2015. Retrieved 20 August 2016.
- ↑ "Is there a cure for Prader-Willi syndrome (PWS)?". NICHD. 2014-01-14. Retrieved 20 August 2016.
- ↑ "What are the treatments for Prader-Willi syndrome (PWS)?". NICHD. 2014-01-14. Retrieved 20 August 2016.
- ↑ Mia, Md Mohan (2016). Classical and Molecular Genetics. American Academic Press. p. 195. ISBN 978-1-63181-776-2.
- ↑ Jorde, Lynn B.; Carey, John C.; Bamshad, Michael J. (2015). Medical Genetics (5 ed.). Elsevier Health Sciences. p. 120. ISBN 978-0-323-18837-1.
- 1 2 3 4 5 Cassidy, S. B., & Driscoll, D. J. (2009). Prader–Willi syndrome. European Journal of Human Genetics, 17(1), 3–13. http://doi.org/10.1038/ejhg.2008.165
- 1 2 Curfs LM, Fryns JP (1992). "Prader-Willi syndrome: a review with special attention to the cognitive and behavioral profile". Birth Defects Orig. Artic. Ser. 28 (1): 99–104. PMID 1340242.
- 1 2 3 Cassidy SB (1997). "Prader-Willi syndrome". Journal of Medical Genetics. 34 (11): 917–23. doi:10.1136/jmg.34.11.917. PMC 1051120
 . PMID 9391886.
. PMID 9391886. - 1 2 3 4 Udwin O (November 1998). "Prader-Willi syndrome: Psychological and behavioural characteristics". Contact a Family.
- ↑ Holm VA, Cassidy SB, Butler MG, Hanchett JM, Greenswag LR, Whitman BY, Greenberg F (1993). "Prader-Willi syndrome: consensus diagnostic criteria". Pediatrics. 91 (2): 398–402. PMID 8424017.
- ↑ Whittington J, Holland A, Webb T, Butler J, Clarke D, Boer H (February 2004). "Cognitive abilities and genotype in a population-based sample of people with Prader-Willi syndrome". J Intellect Disabil Res. 48 (Pt 2): 172–87. doi:10.1111/j.1365-2788.2004.00556.x. PMID 14723659.
- 1 2 3 https://www.nichd.nih.gov/health/topics/prader-willi/conditioninfo/Pages/treatments.aspx
- 1 2 3 Nordqvist, Christian (March 15, 2010). "What Is Prader-Willi Syndrome? What Causes Prader-Willi Syndrome?". Medical News Today. MediLexicon International. Retrieved December 4, 2012.
- ↑ Cummings, D.E., Purnell, J.Q., Vaisse, C., Foster, K.E., Frayo, R.S., Schwartz, M.W., Basdevant, A., & Weigle, D.S. (2002). "Elevated plasma ghrelin levels in Prader Willi syndrome". Nature Medicine. 8: 643–644. doi:10.1038/nm0702-643.
- ↑ Clark DJ, Boer H, Webb T (1995). "General and behavioural aspects of PWS: a review". Mental Health Research. 8 (195): 38–49.
- ↑ Cassidy SB, Devi A, Mukaida C (1994). "Aging in PWS: 232 patients over age 30 years". Proc. Greenwood Genetic Centre. 13: 102–3.
- ↑ Hered RW, Rogers S, Zang YF, Biglan AW (1988). "Ophthalmologic features of Prader-Willi syndrome". J Pediatr Ophthalmol Strabismus. 25 (3): 145–50. PMID 3397859.
- ↑ Online Mendelian Inheritance in Man (OMIM) Prader-Willi Syndrome; PWS -17627
- ↑ de los Santos T, Schweizer J, Rees CA, Francke U (November 2000). "Small evolutionarily conserved RNA, resembling C/D box small nucleolar RNA, is transcribed from PWCR1, a novel imprinted gene in the Prader-Willi deletion region, which Is highly expressed in brain". American Journal of Human Genetics. 67 (5): 1067–82. doi:10.1086/303106. PMC 1288549
 . PMID 11007541.
. PMID 11007541. - ↑ Cavaillé J, Buiting K, Kiefmann M, Lalande M, Brannan CI, Horsthemke B, Bachellerie JP, Brosius J, Hüttenhofer A (December 2000). "Identification of brain-specific and imprinted small nucleolar RNA genes exhibiting an unusual genomic organization". Proc. Natl. Acad. Sci. USA. 97 (26): 14311–6. doi:10.1073/pnas.250426397. PMC 18915
 . PMID 11106375.
. PMID 11106375. - ↑ "Prader-Willi Syndrome - MeSH - NCBI." National Center for Biotechnology Information. U.S. National Library of Medicine, n.d. Web. 01 Nov. 2016. <https://www.ncbi.nlm.nih.gov/mesh/68011218>.
- ↑ Buiting, K; Saitoh, S; Gross, S; Dittrich, B; Schwartz, S; Nicholls, RD; Horsthemke, B (April 1995). "Inherited microdeletions in the Angelman and Prader-Willi syndromes define an imprinting centre on human chromosome 15.". Nature Genetics. 9 (4): 395–400. doi:10.1038/ng0495-395. PMID 7795645.
- ↑ "Major breakthrough in understanding Prader-Willi syndrome, a parental imprinting disorder". Medicalxpress.com. Retrieved 2015-06-18.
- ↑ Runte M, Varon R, Horn D, Horsthemke B, Buiting K (2005). "Exclusion of the C/D box snoRNA gene cluster HBII-52 from a major role in Prader-Willi syndrome.". Hum Genet. 116 (3): 228–30. doi:10.1007/s00439-004-1219-2. PMID 15565282.
- ↑ Skryabin BV, Gubar LV, Seeger B, Pfeiffer J, Handel S, Robeck T, Karpova E, Rozhdestvensky TS, Brosius J (2007). "Deletion of the MBII-85 snoRNA gene cluster in mice results in postnatal growth retardation". PLoS Genet. 3 (12): e235. doi:10.1371/journal.pgen.0030235. PMC 2323313
 . PMID 18166085.
. PMID 18166085. - ↑ Sahoo T, del Gaudio D, German JR, Shinawi M, Peters SU, Person RE, Garnica A, Cheung SW, Beaudet AL (2008). "Prader-Willi phenotype caused by paternal deficiency for the HBII-85 C/D box small nucleolar RNA cluster.". Nat Genet. 40 (6): 719–21. doi:10.1038/ng.158. PMC 2705197
 . PMID 18500341.
. PMID 18500341. - ↑ Ding F, Li HH, Zhang S, Solomon NM, Camper SA, Cohen P, Francke U (2008). Akbarian, Schahram, ed. "SnoRNA Snord116 (Pwcr1/MBII-85) deletion causes growth deficiency and hyperphagia in mice". PLoS ONE. 3 (3): e1709. doi:10.1371/journal.pone.0001709. PMC 2248623
 . PMID 18320030.
. PMID 18320030. - ↑ Ding F, Prints Y, Dhar MS, Johnson DK, Garnacho-Montero C, Nicholls RD, Francke U (2005). "Lack of Pwcr1/MBII-85 snoRNA is critical for neonatal lethality in Prader-Willi syndrome mouse models". Mamm Genome. 16 (6): 424–31. doi:10.1007/s00335-005-2460-2. PMID 16075369.
- ↑ de Smith AJ, Purmann C, Walters RG, Ellis RJ, Holder SE, Van Haelst MM, Brady AF, Fairbrother UL, Dattani M, Keogh JM, Henning E, Yeo GS, O'Rahilly S, Froguel P, Farooqi IS, Blakemore AI (June 2009). "A Deletion of the HBII-85 Class of Small Nucleolar RNAs (snoRNAs) is Associated with Hyperphagia, Obesity and Hypogonadism". Hum. Mol. Genet. 18 (17): 3257–65. doi:10.1093/hmg/ddp263. PMC 2722987
 . PMID 19498035.
. PMID 19498035. - 1 2 Killeen, Anthony A. (2004). "Genetic Inheritance". Principles of Molecular Pathology. Humana Press. p. 41. ISBN 978-1-58829-085-4.
- ↑ Tweed, Katherine (September 2009). "Shawn Cooper Struggles with Prader Willi Syndrome". AOL Health. Retrieved September 2009. Check date values in:
|access-date=(help) - ↑ Davies PS, Evans S, Broomhead S, Clough H, Day JM, Laidlaw A, Barnes ND (May 1998). "Effect of growth hormone on height, weight, and body composition in Prader-Willi syndrome". Arch. Dis. Child. 78 (5): 474–6. doi:10.1136/adc.78.5.474. PMC 1717576
 . PMID 9659098.
. PMID 9659098. - ↑ Carrel AL, Myers SE, Whitman BY, Allen DB (April 2002). "Benefits of long-term GH therapy in Prader-Willi syndrome: a 4-year study". J. Clin. Endocrinol. Metab. 87 (4): 1581–5. doi:10.1210/jc.87.4.1581. PMID 11932286.
- ↑ Höybye C, Hilding A, Jacobsson H, Thorén M (May 2003). "Growth hormone treatment improves body composition in adults with Prader-Willi syndrome". Clin. Endocrinol. (Oxf). 58 (5): 653–61. doi:10.1046/j.1365-2265.2003.01769.x. PMID 12699450.
- ↑ "Double-Blind, Placebo Controlled, Phase 3 Trial of ZGN-440 (Beloranib) in Obese Subjects With Prader-Willi Syndrome – Full Text View – ClinicalTrials.gov". Retrieved March 24, 2016.
- ↑ "Potential PWS Treatments Currently in Development". Foundation for Prader-Willi Research. Retrieved March 24, 2016.
- ↑ "Unmet Needs in Prader-Willi Syndrome". Retrieved March 24, 2016.
- ↑ "An Efficacy, Safety, and Pharmacokinetics Study of Beloranib in Obese Subjects With Prader-Willi Syndrome". Retrieved March 24, 2016.
- ↑ "Zafgen Announces Initial Results from Phase 2a Study of Beloranib in Patients with Prader-Willi Syndrome". Retrieved March 24, 2016.
- ↑ "UPDATE 4-Zafgen halts obesity drug trial after second patient death". Reuters. 2015-12-03. Retrieved 2016-02-26.
- ↑ Mary Jones. "Case Study: Cataplexy and SOREMPs Without Excessive Daytime Sleepiness in Prader Willi Syndrome. Is This the Beginning of Narcolepsy in a Five Year Old?". European Society of Sleep Technologists. Retrieved April 6, 2009.
- ↑ "Dog Eat Dog". Csifiles.com. Retrieved June 12, 2009.
- ↑ "Can't Stop Eating". Channel4.com. 2006. Retrieved June 12, 2009.
- ↑ "Extreme Makeover: Home Edition Articles on AOL TV". Aoltv.com. Retrieved 2015-06-18.
- ↑ Archived July 14, 2014, at the Wayback Machine.
- ↑ Group urges more support for Prader-Willi sufferers, Taipei Times. Published December 24, 2011. Retrieved May 27, 2012.
Cassidy, S. B., & Driscoll, D. J. (2009). Prader–Willi syndrome. European Journal of Human Genetics, 17(1), 3–13. http://doi.org/10.1038/ejhg.2008.165
External links
| Wikimedia Commons has media related to Prader-Willi syndrome. |