Rhenium trioxide
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Rhenium trioxide | |||
| Other names
Rhenia | |||
| Identifiers | |||
| 1314-28-9 | |||
| 3D model (Jmol) | Interactive image | ||
| ECHA InfoCard | 100.013.845 | ||
| EC Number | 215-228-8 | ||
| PubChem | 102110 | ||
| |||
| |||
| Properties | |||
| ReO3 | |||
| Molar mass | 234.205 g/mol | ||
| Appearance | Deep red crystals | ||
| Density | 6.92 g/cm3 | ||
| Melting point | 400 °C (752 °F; 673 K) (decomposes) | ||
| Refractive index (nD) |
1.68 | ||
| Structure | |||
| Cubic, cP4 | |||
| Pm3m, SpaceGroup = 221 | |||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| | |||
| Infobox references | |||
Rhenium trioxide or rhenium(VI) oxide is an inorganic compound with the formula ReO3. It is a red solid with a metallic lustre. It is the only stable trioxide of the Group 7 elements (Mn, Tc, Re).
Preparation, structure
Rhenium trioxide can be formed by reducing rhenium(VII) oxide with carbon monoxide. [1]
- Re2O7 + CO → 2 ReO3 + CO2
Re2O7 can also be reduced with dioxane.[2]
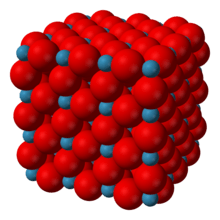
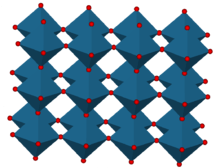
Rhenium oxide crystallizes with a primitive cubic unit cell, with a lattice parameter of 3.742 Å (374.2 pm). The structure of ReO3 is similar to that of perovskite (ABO3), without the large A cation at the centre of the unit cell. Each rhenium center is surrounded by an octahedron defined by six oxygen centers. These octahedra share corners to form the 3-dimensional structure. The coordination number of O is 2 because each oxygen atom has 2 neighbouring Re atoms.[3]
Properties
Upon heating to 400 °C under vacuum, it undergoes disproportionation:[2]
- 3 ReO3 → Re2O7 + ReO2
ReO3 is unusual for an oxide because it exhibits very low resistivity. It behaves like a metal in that its resistivity decreases as its temperature decreases. At 300 K, its resistivity is 100.0 nΩ·m, whereas at 100 K, this decreases to 6.0 nΩ·m, 17 times less than at 300 K.[3]
Uses
Hydrogenation Catalyst
Rhenium trioxide finds some use in organic synthesis as a catalyst for amide reduction.[4]
References
- ↑ H. Nechamkin, C. F. Hiskey, "Rhenium(VI): Oxide (Rhenium Trioxide)" Inorganic Syntheses, 1950 Volume 3, pp. 186-188. doi:10.1002/9780470132340.ch49
- 1 2 G. Glemser "Rhenium (VI) Oxide" Handbook of Preparative Inorganic Chemistry, 2nd Ed. Edited by G. Brauer, Academic Press, 1963, NY. Vol. 2. p. 1482.
- 1 2 Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 0-08-037941-9., p. 1047.
- ↑ Nishimura, Shigeo (2001). Handbook of Heterogeneous Catalytic Hydrogenation for Organic Synthesis (1st ed.). Newyork: Wiley-Interscience. p. 408. ISBN 9780471396987.
