Sodium tungsten bronze

Sodium tungsten bronze is a form of insertion compound with the formula NaxWO3, where x is equal to or less than 1. Named due to its metallic lustre, its electrical properties range from semiconducting to metallic depending on the concentration of sodium ions present; it can also exhibit superconductivity.
History
Prepared in 1823 by the chemist Friedrich Wöhler, sodium tungsten bronze was the first alkali metal bronze to be discovered.[1] They owe some of their properties to the relative stability of the tungsten(V) cation that is formed.[2] A similar family of molybdenum bronzes may have been discovered in 1885 by Alfred Stavenhagen and E. Engels,[3] but they are formed in a very narrow range of temperatures and were not reported again until the 1960s.[4]
Properties
Sodium tungsten bronze, like other tungsten bronzes, is resistant to chemical reaction under both acidic and basic conditions. Colour is dependent upon the proportion of sodium in the compound, ranging from golden at x≈0.9, through red, orange and deep purple, to blue-black when x≈0.3.
The electrical resistivity of the bronze depends on the proportion of sodium in the compound, with specific resistances of 1.66 mΩ being measured for some samples.[5] It has been suggested that electrons, released when the sodium atoms are ionised, are conducted readily through the tungsten t2g and oxygen π orbitals.[2] This can be observed in the XPS[6] and UPS[7] spectra: the peak representing the tungsten 5d band becomes more intense as x rises.
For values of x below 0.3, the bronze is semiconducting rather than metallic.[2] When cooled sufficiently, sodium tungsten bronze becomes a superconductor, with the critical temperature (Tc) for Na0.23WO3 being approximately 2.2 kelvin.[8] The first record of superconductivity in a tungsten bronze was in 1964, with a Tc of 0.57 K.[9]
Structure
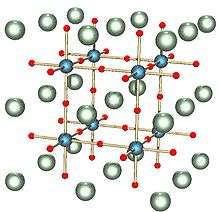
When x=1, sodium tungsten bronze adopts a cubic phase: the perovskite crystal structure.[10] In this form, the structure consists of corner-sharing WO6 octahedra with sodium ions in the interstitial gaps. For x values between 0.9 and 0.3, the structure remains similar but with an increasing deficiency of sodium ions and a smaller lattice parameter.[10]
A number of other structure types can also be adopted, with varying electrical properties: cubic, tetragonal I and hexagonal phases are metallic, whereas orthorhombic and tetragonal II structures are semiconducting.[11]
Synthesis
Wöhler's 1823 synthesis involved reducing sodium tungstate and tungsten trioxide with hydrogen gas at red heat. A more modern approach reduces a melt of the reactants with electricity rather than with hydrogen.[12] Microwave synthesis is also possible,[13] using tungsten powder as the reducing agent. Hydrothermal (both batch and flow) syntheses are also possible.[14]
Related compounds
The sodium in this compound can be replaced by other alkali metals to form their tungsten bronzes, and by other metals such as tin and lead.[15] Molybdenum bronzes also exist but are less stable than their tungsten counterparts.[2]
References
- ↑ Hagenmuller, P (1973). "Chapter 50: Tungsten bronzes, vanadium bronzes, and related compounds". Comprehensive Inorganic Chemistry. 4. Pergamon. pp. 541–605. ISBN 978-0-08-016989-7.
- 1 2 3 4 Greenwood, N. N. & Earnshaw, A. (1993) [Reprint of corrected version of 1986, original version printed 1984]. Chemistry of the Elements (1st ed.). Pergamon Press. pp. 1185–6. ISBN 0-08-022057-6.
- ↑ A. Stavenhagen, E. Engels (1895) "Ueber Molybdänbronzen" Berichte der deutschen chemischen Gesellschaft, volume 28, pages 2280-2281. doi:10.1002/cber.189502802213
- ↑ A. Wold, W. Kunnmann, R. J. Arnott, and A. Ferreti (1964), "Preparation and properties of sodium and potassium molybdenum bronze crystals". Inorganic Chemistry, volume 3, issue 4, pages 545-547. doi:10.1021/ic50014a022
- ↑ Straumanis, M. E.; Dravnieks, A. (1949). "The Sodium Tungsten Bronzes. II. The Electrical Conductivity of the Bronzes". Journal of the American Chemical Society. 71 (2): 683. doi:10.1021/ja01170a086.
- ↑ West, Anthony (1984). Solid state chemistry and its applications. Wiley. p. 96. ISBN 0-471-90874-6.
- ↑ Cheetham, A K.; Day, P. (1987). Solid state chemistry: techniques. Clarendon. p. 110. ISBN 0-19-855286-6.
- ↑ Ostenson, J.; Shanks, H.; Finnemore, D. (1978). "Superconductivity in the tungsten bronzes". Journal of the Less Common Metals. 62: 149. doi:10.1016/0022-5088(78)90024-3.
- ↑ Raub, C.; Sweedler, A.; Jensen, M.; Broadston, S.; Matthias, B. (1964). "Superconductivity of Sodium Tungsten Bronzes". Physical Review Letters. 13 (25): 746. Bibcode:1964PhRvL..13..746R. doi:10.1103/PhysRevLett.13.746.
- 1 2 Hägg, G. (1935). "The Spinels and the Cubic Sodium-Tungsten Bronzes as New Examples of Structures with Vacant Lattice Points". Nature. 135 (3421): 874. Bibcode:1935Natur.135..874H. doi:10.1038/135874b0.
- ↑ Ngai, K. L.; Reinecke, T. L. (1978). "Structural instabilities and superconductivity in the alkali tungsten bronzes". Journal of Physics F: Metal Physics. 8: 151. Bibcode:1978JPhF....8..151N. doi:10.1088/0305-4608/8/1/018.
- ↑ Conroy, L. E. (1977). "The preparation and characterization of a sodium tungsten bronze. An inorganic experiment". Journal of Chemical Education. 54: 45–34. Bibcode:1977JChEd..54...45C. doi:10.1021/ed054p45.
- ↑ Guo, J.; Dong, C.; Yang, L.; Fu, G. (2005). "A green route for microwave synthesis of sodium tungsten bronzes NaWO (0<<1)". Journal of Solid State Chemistry. 178: 58. Bibcode:2005JSSCh.178...58G. doi:10.1016/j.jssc.2004.10.017.
- ↑ Luo, Jia Yu; Liu, Jing Xiao; Shi, Fei; Xu, Qiang; Jiang, Yan Yan; Liu, Gui Shan; Hu, Zhi Qiang (June 2013). "Synthesis of Sodium Tungsten Bronze via Hydrothermal Method Assisted by Citric Acid". Advanced Materials Research. 712-715: 280–283. doi:10.4028/www.scientific.net/AMR.712-715.280.
- ↑ Smart, Lesley E.; Moore, Elaine A. (2005). Solid State Chemistry: An Introduction (3rd ed.). CRC Press. p. 227. ISBN 0-7487-7516-1.