Anaplastic large-cell lymphoma
| Anaplastic large-cell lymphoma | |
|---|---|
|
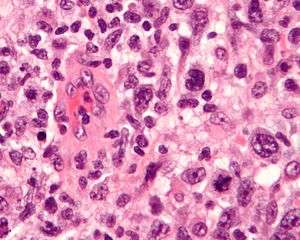
Micrograph of an anaplastic large cell lymphoma. H&E stain. | |
| Classification and external resources | |
| Specialty | Hematology and oncology |
| ICD-10 | C84.6, C84.7 |
| ICD-9-CM | 200.6 |
| ICD-O | M9714/3 |
| eMedicine | derm/534 |
| MeSH | D017728 |
Anaplastic large-cell lymphoma (ALCL) is a type of non-Hodgkin lymphoma involving aberrant T-cells. It is described in detail in the "Classification of Tumors of the Hematopoietic and Lymphoid Tissues" edited by experts of the World Health Organisation (WHO). The term anaplastic large cell lymphoma (ALCL) encompasses at least 4 different clinical entities, all sharing the same name, and histologically have also in common the presence of large pleomorphic cells that express CD30 and T-cell markers. Two types of ALCL present as systemic disease and are considered as aggressive lymphomas, while two types present as localized disease and may progress locally.
Its name derives from anaplasia and large-cell lymphoma.
Signs and symptoms
The clinical presentation varies according to the type of ALCL. Two of the ALCL are systemic lymphomas, in that usually present with enlarged lymph nodes in multiple regions of the body, or with tumors outside the lymph nodes (extranodal) such as bone, intestine, muscle, liver, or spleen. These 2 subtypes usually associate with weight loss, fevers and night sweats, and can be lethal if left untreated without chemotherapy.[1] The third type of ALCL is so-called cutaneous ALCL, and is a tumor that presents in the skin as ulcers that may persist, or occasionally may involute spontaneously, and commonly recur. This type of ALCL usually manifest in different regions of the body and may extend to regional lymph nodes, i.e., an axillary lymph node if the ALCL presents in the arm.[2] A more recently recognized subtype of ALCL is that associated with breast implants. The tumor initially manifests with swelling of the breast due to fluid accumulation around the implant. The disease may progress to invade the tissue surrounding the capsule, and if left untreated may progress to axillary lymph nodes.[3]
It typically presents at a late stage and is often associated with systemic symptoms ("B symptoms").
Diagnosis
The diagnosis of ALCL requires the examination by a pathologist of any enlarged lymph node, or any affected extranodal tissue where there the tumor is found, such as the intestine, the liver or bone in the case of systemic ALCL. For the case of cutaneous ALCL, a skin excision is recommended, and for the diagnosis of ALCL associated with breast implants, a cytologic specimen of the effusion around the breast implant or complete examination of the breast capsule surrounding the implant is required.[4]
To make this diagnosis under its present system of classification, the WHO emphasizes the identification of "hallmark" cells and immunopositivity for CD30. Integration of this information with clinical presentation is crucial for final classification and management of patients.
The classification acknowledges the recognition of large cells with pleomorphic nuclei and abundant cytoplasm. The morphologic features require to complement with immunophenotypic evidence that cells are T lymphocytes, such as the expression of immunologic markers CD3 or CD4, but always it is required the expression of CD30 in all neoplastic cells. Out of the 4 types of ALCL, one subtype of systemic ALCL expresses the protein anaplastic lymphoma kinase (ALK), and the other types of ALCL do not express ALK.
The hallmark cells are of medium size and feature abundant cytoplasm (which may be clear, amphophilic or eosinophilic), kidney shaped nuclei, and a paranuclear eosinophilic region. Occasional cells may be identified in which the plane of section passes through the nucleus in such a way that it appears to enclose a region of cytoplasm within a ring; such cells are called "doughnut" cells.
By definition, on histological examination, hallmark cells are always present. Where they are not present in large numbers, they are usually located around blood vessels. Morphologic variants include the following types:
- Common (featuring a predominance of hallmark cells)
- Small-cell (featuring smaller cells with the same immunophenotype as the hallmark cells)
- Lymphohistiocytic
- Sarcomatoid
- Signet ring
Immunophenotype
The hallmark cells (and variants) show immunopositivity for CD30[5][6] (also known as Ki-1). True positivity requires localisation of signal to the cell membrane and/or paranuclear region (cytoplasmic positivity is considered non-specific and non-informative). Another useful marker which helps to differentiate this lesion from Hodgkin lymphoma is Clusterin. The neoplastic cells have a golgi staining pattern (hence paranuclear staining), which is characteristic of this lymphoma. The cells are also typically positive for a subset of markers of T-cell lineage. However, as with other T-cell lymphomas, they are usually negative for the pan T-cell marker CD3. Occasional examples are of null (neither T nor B) cell type. These lymphomas show immunopositivity for ALK protein in 70% of cases. They are also typically positive for EMA. In contrast to many B-cell anaplastic CD30 positive lymphomas, they are negative for markers of Epstein–Barr virus (EBV).
Molecular biology
The majority of cases, greater than 90%, contain a clonal rearrangement of the T-cell receptor. This may be identified using PCR techniques, such as T-gamma multiplex PCR. Oncogeneic potential is conferred by upregulation of a tyrosine kinase gene on chromosome 2. Several different translocations involving this gene have been identified in different cases of this lymphoma. The most common is a chromosomal translocation involving the nucleophosmin gene on chromosome 5. The translocation may be identified by analysis of giemsa-banded metaphase spreads of tumour cells and is characterised by t(2;5)(p23;q35). The product of this fusion gene may be identified by immunohistochemistry using antiserum to ALK protein. Probes are available to identify the translocation by fluorescent in situ hybridization. The nucleophosmin component associated with the commonest translocation results in nuclear positivity as well as cytoplasmic positivity. Positivity with the other translocations may be confined to the cytoplasm. Mutagenesis and functional studies have identified a plethora of NPM1–ALK interacting molecules which ultimately lead to the activation of key pathways including RAS/Erk, PLC-γ, PI3K, and Jak/signal transducers and activators of transcription (STAT) path- ways, which in turn control cell proliferation and survival and cytoskeletal rearrangements.[7] It has been demonstrated that NPM-ALK oncogenic effects is sustained by STAT3 activation. Activation of STAT3 is associated with a specific signature, which includes several transcription factors (i.e., CEBP/β), cell cycle (i.e., Cyclin D, c-myc etc.), survival/apoptosis molecules (Bcl-A2, Bcl-XL, Survivin, MCL-1) and cell adhesion, and mobility proteins.[8]
Differential diagnosis and diagnostic pitfalls
As the appearance of the hallmark cells, pattern of growth (nesting within lymph nodes) and positivity for EMA may mimic metastatic carcinoma, it is important to include markers for cytokeratin in any diagnostic panel (these will be negative in the case of anaplastic lymphoma). Other mimics include CD30 positive B-cell lymphomas with anaplastic cells (including Hodgkin lymphomas). These are identified by their positivity for markers of B-cell lineage and frequent presence of markers of EBV. Primary cutaneous T-cell lymphomas may also be positive for CD30; these are excluded by their anatomic distribution. ALK positivity may also be seen in some large-cell B-cell lymphomas and occasionally in rhabdomyosarcomas.
Treatment
- For the cases of systemic ALCL, the recommendation is to Manage under "Aggressive Lymphoma" guidelines.
- CHOP is first line of treatment, CHOP-Rituximab in the unlikely scenario that CD20 is positive, given that CD20 is a B-cell marker.
- Radiation therapy as per institutional preference (based on ECOG, SWOG, and GELA trials), but usually added for bulky disease
- Localized primary cutaneous ALCL[9]
Breast implant-associated ALCL is a recently recognized lymphoma and definitive management and therapy is under evaluation. However, it appears that removal of the implant, and resection of the capsule around the implant as well as evaluation by medical and surgical oncologists are cornerstones. Still under evaluation is the extent of capsulectomy: partial versus complete capsulectomy; similarly it is not defined the significance of replacement of the implant in the affected breast, or the removal of contralateral implant. Similarly, the value of radiation therapy and chemotherapy are under evaluation.
- Radiation therapy alone
- Surgical excision alone
- Surgically excision with adjuvant radiation.
Currently, there is a drug, LDK378, undergoing Phase III clinical trials at Vanderbilt University that targets ALK positive small cell lung cancer,[10] and has showed clinical promise in its previous clinical trials.[11] Because approximately 70% of ALCL neoplasms are also ALK positive, there is hope that similar highly selective and potent ALK inhibitors may be used in the future to treat ALK positive cases of ALCL.[12][13]
Prognosis
The prognosis varies according with the type of ALCL. During treatment, relapses may occur but these typically remain sensitive to chemotherapy.
Those with ALK positivity have better prognosis than ALK negative ALCL. It has been suggested that ALK-negative anaplastic large-cell lymphomas derive from other T-cell lymphomas that are morphologic mimics of ALCL in a final common pathway of disease progression. Whereas ALK-positive ALCLs are molecularly characterized and can be readily diagnosed, specific immunophenotypic or genetic features to define ALK-negative ALCL are missing and their distinction from other T-cell non-Hodgkin lymphomas (T-NHLs) remains controversial, although promising diagnostic tools for their recognition have been developed and might be helpful to drive appropriate therapeutic protocols.[14]
Systemic ALK+ ALCL 5-year survival: 70–80%.[9] Systemic ALK- ALCL 5-year survival: 15–45%.[9] Primary Cutaneous ALCL: Prognosis is good if there is not extensive involvement regardless of whether or not ALK is positive with an approximately 90% 5-year survival rate.[9] Breast implant-associated ALCL has an excellent prognosis when the lymphoma is confined to the fluid or to the capsule surrounding the breast implant. This tumor can be recurrent and grow as a mass around the implant capsule or can extend to regional lymph nodes if not properly treated.[4]
Epidemiology
The lymphoma is more common in the young and in males.
A 2008 study found an increased risk of ALCL of the breast in women with silicone breast implants, although the overall risk remained exceedingly low due to the rare occurrence of the tumor.[15]
References
- ↑ Medeiros LJ, Elenitoba-Johnson KS. Anaplastic Large Cell Lymphoma. Am J Clin Pathol. 2007 May;127(5):707–22.
- ↑ Kempf W, Pfaltz K, Vermeer MH, Cozzio A, Ortiz-Romero PL, Bagot M, Olsen E, Kim YH, Dummer R, Pimpinelli N, Whittaker S, Hodak E, Cerroni L, Berti E, Horwitz S, Prince HM, Guitart J, Estrach T, Sanches JA, Duvic M, Ranki A, Dreno B, Ostheeren-Michaelis S, Knobler R, Wood G, Willemze R. EORTC, ISCL, and USCLC consensus recommendations for the treatment of primary cutaneous CD30-positive lymphoproliferative disorders: lymphomatoid papulosis and primary cutaneous anaplastic large-cell lymphoma. Blood. 2011 Oct 13;118(15):4024–35.
- ↑ Miranda RN, Aladily TN, Prince HM, Kanagal-Shamanna R, de Jong D, Fayad LE,Amin MB, Haideri N, Bhagat G, Brooks GS, Shifrin DA, O'Malley DP, Cheah CY, Bacchi CE, Gualco G, Li S, Keech JA Jr, Hochberg EP, Carty MJ, Hanson SE, Mustafa E, Sanchez S, Manning JT Jr, Xu-Monette ZY, Miranda AR, Fox P, Bassett RL, Castillo JJ, Beltran BE, de Boer JP, Chakhachiro Z, Ye D, Clark D, Young KH, Medeiros LJ. Breast implant-associated anaplastic large-cell lymphoma: long-term follow-up of 60 patients. J Clin Oncol. 2014 Jan 10;32(2):114–20.
- 1 2 Miranda RN, Aladily TN, Prince HM, Kanagal-Shamanna R, de Jong D, Fayad LE, Amin MB, Haideri N, Bhagat G, Brooks GS, Shifrin DA, O'Malley DP, Cheah CY, Bacchi CE, Gualco G, Li S, Keech JA Jr, Hochberg EP, Carty MJ, Hanson SE, Mustafa E, Sanchez S, Manning JT Jr, Xu-Monette ZY, Miranda AR, Fox P, Bassett RL, Castillo JJ, Beltran BE, de Boer JP, Chakhachiro Z, Ye D, Clark D, Young KH, Medeiros LJ. Breast implant-associated anaplastic large-cell lymphoma: long-term follow-up of 60 patients. J Clin Oncol. 2014 Jan 10;32(2):114–20.
- ↑ Watanabe M, Ogawa Y, Itoh K, et al. (January 2008). "Hypomethylation of CD30 CpG islands with aberrant JunB expression drives CD30 induction in Hodgkin lymphoma and anaplastic large cell lymphoma". Lab. Invest. 88 (1): 48–57. doi:10.1038/labinvest.3700696. PMID 17965727.
- ↑ Park SJ, Kim S, Lee DH, et al. (August 2008). "Primary systemic anaplastic large cell lymphoma in Korean adults: 11 years' experience at Asan Medical Center". Yonsei Med. J. 49 (4): 601–9. doi:10.3349/ymj.2008.49.4.601. PMC 2615286
 . PMID 18729302.
. PMID 18729302. - ↑ Tabbó F, Barreca A, Piva R, Inghirami G, European T-Cell Lymphoma Study Group (2012). "ALK Signaling and Target Therapy in Anaplastic Large Cell Lymphoma". Front Oncol. 2: 41. doi:10.3389/fonc.2012.00041. PMC 3355932
 . PMID 22649787.
. PMID 22649787. - ↑ Piva R, Agnelli L, Pellegrino E, Todoerti K, et al. (2010). "Gene expression profiling uncovers molecular classifiers for the recognition of anaplastic large-cell lymphoma within peripheral T-cell neoplasms". J Clin Oncol. 28 (9): 1583–90. doi:10.1200/JCO.2008.20.9759. PMID 20159827.
- 1 2 3 4 Liu, Delong. "Anaplastic Large Cell Lymphoma". Medscape.
- ↑ http://www.vicc.org/ct/protocol-results.php?protocol-no=VICCTHN1332
- ↑ http://www.novartis.com/newsroom/media-releases/en/2013/1706664.shtml
- ↑ http://www.targetedonc.com/publications/targeted-therapies-cancer/14/December-2013/LDK378-A-Promising-Next-Generation-ALK-Inhibitor-With-FDA-Breakthrough-Therapy-Designation
- ↑ https://pct.mdanderson.org/genes/alk/show
- ↑ Agnelli L, Mereu E, Pellegrino E, Limongi T, et al. (2012). "Identification of a 3-gene model as a powerful diagnostic tool for the recognition of ALK-negative anaplastic large-cell lymphoma". Blood. 120 (6): 1274–81. doi:10.1182/blood-2012-01-405555. PMID 22740451.
- ↑ de Jong D, Vasmel WL, de Boer JP, et al. (November 2008). "Anaplastic large-cell lymphoma in women with breast implants". JAMA. 300 (17): 2030–5. doi:10.1001/jama.2008.585. PMID 18984890.
External links
- Information on Anaplastic Large Cell (Ki-1 / CD-30) Lymphomas from Lymphoma Information Network