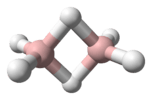Beryllium hydride
 | |
| Names | |
|---|---|
| Other names
Beryllium dihydride Beryllium hydride Beryllane | |
| Identifiers | |
| 7787-52-2 | |
| 3D model (Jmol) | Interactive image |
| ChEBI | CHEBI:33787 |
| ChemSpider | 17215712 |
| PubChem | 139073 |
| |
| |
| Properties | |
| BeH2 | |
| Molar mass | 11.03 g mol−1 |
| Appearance | amorphous white solid[1] |
| Density | 0.65 g/cm3 |
| Melting point | 250 °C (482 °F; 523 K) decomposes |
| decomposes | |
| Solubility | insoluble in diethyl ether, toluene |
| Thermochemistry | |
| 30.124 J/mol K | |
| Hazards | |
| US health exposure limits (NIOSH): | |
| PEL (Permissible) |
TWA 0.002 mg/m3 C 0.005 mg/m3 (30 minutes), with a maximum peak of 0.025 mg/m3 (as Be)[2] |
| REL (Recommended) |
Ca C 0.0005 mg/m3 (as Be)[2] |
| IDLH (Immediate danger) |
Ca [4 mg/m3 (as Be)][2] |
| Related compounds | |
| Other cations |
lithium hydride, calcium hydride, boron hydrides |
| Related compounds |
beryllium fluoride |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
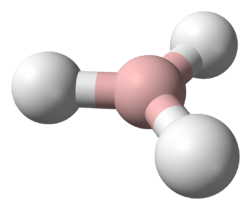
Beryllium hydride (systematically named beryllium dihydride) is an inorganic compound with the chemical formula (BeH
2)n (also written ([BeH
2])n or BeH
2). This alkaline earth hydride is a colourless solid that is insoluble in solvents that do not decompose it.[3] Unlike the ionically bonded hydrides of the heavier Group 2 elements, beryllium hydride is covalently bonded[1] (three-center two-electron bond).
Synthesis
Unlike the other group 2 metals, beryllium does not react with hydrogen.[4] Instead, BeH2 is prepared from preformed beryllium(II) compounds. It was first synthesised in 1951 by treating dimethylberyllium, Be(CH3)2, with lithium aluminium hydride, LiAlH4.[5]
Purer BeH2 forms from the pyrolysis of di-tert-butylberyllium, Be(C(CH3)3)2 at 210 °C.[6]
A route to highly pure samples involve the reaction of triphenylphosphine, PPh3, with beryllium borohydride, Be(BH4)2:[1]
- Be(BH4)2 + 2 PPh3 → 2 Ph3PBH3 + BeH2
Structure
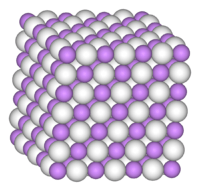
BeH2 is usually formed as an amorphous white solid, but a hexagonal crystalline form with a higher density (~0.78 g cm−3) was reported,[7] prepared by heating amorphous BeH2 under pressure, with 0.5-2.5% LiH as a catalyst.
A more recent investigation found that crystalline beryllium hydride has a body-centred orthorhombic unit cell, containing a network of corner-sharing BeH4 tetrahedra, in contrast to the flat, hydrogen-bridged, infinite chains previously thought to exist in crystalline BeH2.[8]
Studies of the amorphous form also find that it consists of a network of corner shared tetrahedra.[9]
Chemical properties
Reaction with water and acids
Beryllium hydride reacts slowly with water but is rapidly hydrolysed by acid such as hydrogen chloride to form beryllium chloride.[4]
Reaction with Lewis bases
Beryllium hydride reacts with trimethylamine, N(CH3)3 to form a dimeric aduct, with bridging hydrides.[10] However, with dimethylamine, HN(CH3)2 it forms a trimeric beryllium diamide, [Be(N(CH3)2)2]3 and hydrogen.[4] The reaction with lithium hydride where the hydride ion is the Lewis base, forms sequentially LiBeH3 and Li2BeH4.[4]
Dihydridoberyllium
Dihydridoberyllium is a related compound with the chemical formula BeH
2 (also written [BeH
2]). It is a gas that cannot persist undiluted. Unsolvated dihydridoberyllium will spontaneously autopolymerise to oligomers. Free molecular BeH2 produced by electrical discharge at high temperature has been confirmed as linear with a Be-H bond length of 133.376 pm. Its hybridisation is sp.
[11]
Chemical properties
In theory, the two-coordinate hydridoberyllium group (-BeH) in hydridoberylliums such as dihydridoberyllium can accept an electron-pair donating ligand into the molecule by adduction:[12]
- [BeH
2] + L → [BeH
2L]
Because of this acceptance of the electron-pair donating ligand (L), dihydridoberyllium has Lewis-acidic-acidic character. Dihydridoberyllium can accept four two electron-pairs from ligands, as in the case of the tetrahydridoberyllate(2-) anion (BeH2−
4).
References
- 1 2 3 Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 0-08-037941-9., p. 115
- 1 2 3 "NIOSH Pocket Guide to Chemical Hazards #0054". National Institute for Occupational Safety and Health (NIOSH).
- ↑ Pradyot Patnaik. Handbook of Inorganic Chemicals. McGraw-Hill, 2002, ISBN 0-07-049439-8
- 1 2 3 4 Egon Wiberg, Arnold Frederick Holleman (2001) Inorganic Chemistry, Elsevier ISBN 0-12-352651-5, p. 1048
- ↑ Glenn D. Barbaras; Clyde Dillard; A. E. Finholt; Thomas Wartik; K. E. Wilzbach & H. I. Schlesinger (1951). "The Preparation of the Hydrides of Zinc, Cadmium, Beryllium, Magnesium and Lithium by the Use of Lithium Aluminum Hydride". J. Am. Chem. Soc. 73 (10): 4585–4590. doi:10.1021/ja01154a025.
- ↑ G. E. Coates & F. Glockling (1954). "Di-tert.-butylberyllium and beryllium hydride". J. Chem. Soc.: 2526–2529. doi:10.1039/JR9540002526.
- ↑ G. J. Brendel; E. M. Marlett & L. M. Niebylski (1978). "Crystalline beryllium hydride". Inorg. Chem. 17 (12): 3589–3592. doi:10.1021/ic50190a051.
- ↑ Gordon S. Smith; Quintin C. Johnson; Deane K. Smith; D. E. Cox; Robert L. Snyder; Rong-Sheng Zhou & Allan Zalkin (1988). "The crystal and molecular structure of beryllium hydride". Solid State Communications. 67 (5): 491–494. doi:10.1016/0038-1098(84)90168-6.
- ↑ Sujatha Sampath; Kristina M. Lantzky; Chris J. Benmore; Jörg Neuefeind & Joan E. Siewenie (2003). "Structural quantum isotope effects in amorphous beryllium hydride". J. Chem. Phys. 119 (23): 12499. doi:10.1063/1.1626638.
- ↑ Shepherd Jr., Lawrence H.; Ter Haar, G. L.; Marlett, Everett M. (April 1969). "Amine complexes of beryllium hydride" (PDF). Inorganic Chemistry. American Chemical Society. 8 (4): 976–979. doi:10.1021/ic50074a051. Retrieved 16 October 2013.
- ↑ Peter F. Bernath; Alireza Shayesteh; Keith Tereszchuk; Reginald Colin (2002). "The Vibration-Rotation Emission Spectrum of Free BeH2". Science. 297 (5585): 1323–1324. doi:10.1126/science.1074580. PMID 12193780.
- ↑ Sharp, Stephanie B.; Gellene, Gregory I. (23 November 2000). "σ Bond Activation by Cooperative Interaction with ns2 Atoms: Be + n H
2, n = 1−3". The Journal of Physical Chemistry A. ACS Publications. 104 (46): 10951–10957. doi:10.1021/jp002313m.