Mercury(II) hydride
| Names | |
|---|---|
| IUPAC name
Mercury(II) hydride | |
| Other names
Mercurane Mercuric hydride | |
| Identifiers | |
| 3D model (Jmol) | Interactive image |
| |
| |
| Properties | |
| HgH 2 | |
| Molar mass | 202.61 g mol−1 |
| Related compounds | |
| Related compounds |
Zinc hydride |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
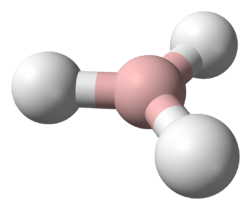
Mercury(II) hydride (systematically named mercurane(2) and dihydridomercury) is an inorganic compound with the chemical formula HgH
2 (also written as [HgH
2]). While its appearance at standard temperature and pressure is unknown, it can present as a white solid, which is thermally stable below −125 °C (−193 °F). The vapour is photosensitive and colourless, with few other known qualitative descriptors. It has no economic uses, and is only intentionally produced for academic reasons. It is investigated as an intermediate chemical species in the reduction of mercuric solutions to elemental mercury, and for its effect on high sensitivity isotope-ratio mass spectrometry methods that involve mercury, such as MC-ICP-MS, when used to compare thallium to mercury.[1]
History
It is suspected that in 1951, mercury(II) hydride was synthesised for the first time by Wiberg et al, by the ethereal reaction of mercury(II) iodide and lithium tetrahydroaluminate. In 1993 Legay-Sommaire announced HgH2 production in cryogenic argon and krypton matrices with a KrF laser.[2] In 2004, solid HgH2 was definitively synthesised and consequentially analysed, by Xuefeng Wang and Lester Andrews, by direct matrix isolation reaction of excited mercury with molecular hydrogen.[3] In 2005, gaseous HgH2 was synthesised by Alireza Shayesteh et al, by the direct gas-phase reaction of excited mercury with molecular hydrogen at standard temperature;[4] and Xuefeng Wang and Lester Andrews[3] determined the structure of solid mercury HgH2, to be a molecular solid.
Chemical properties
Acidity
The two-coordinate hydridomercury group (-HgH) in hydridomercury complexes such as mercury(II) hydride can accept an electron-pair donating ligand into the molecule by adduction:[3]
- [HgH
2] + L → [HgH
2L]
Because of this acceptance of the electron-pair donating ligand (L), mercury(II) hydride has Lewis-acidic character.
Structure
In solid mercury(II) hydride, the HgH2 molecules are connected by mercurophilic bonds. Trimers and a lesser proportion of dimers are detected in the vapour. Unlike solid zinc(II), and cadmium(II) hydride, which are network solids, solid mercury(II) hydride is a covalently bound molecular solid. This is due to relativistic effects, which also accounts for the relatively low decomposition temperature of -125 °C.[5]
The HgH2 molecule is linear and symmetric in the form H-Hg-H. The bond length is 1.646543 Å. The antisymmetric stretching frequency, ν3 of the bond is 1912.8 cm−1, 57.34473 THz for isotopes 202Hg and 1H.[5] The energy needed to break the Hg-H bond in HgH2 is 70 kcal/mol. The second bond in the resulting HgH is much weaker only needing 8.6 kcal/mol to break. Reacting two hydrogen atoms releases 103.3 kcal/mol, and so HgH2 formation from hydrogen molecules and Hg gas is endothermic at 24.2 kcal/mol.[5]
Chemical reactions
Mercury(II) hydride undergoes the typical chemical reactions of a molecular metal hydride. Upon treatment with a standard acid, mercury(II) hydride converts to a mercury salt and elemental hydrogen. Oxidation of mercury(II) hydride gives mercury(II) oxide. When heated above −124 °C (−191 °F), it decomposes into elemental mercury and hydrogen:[3]
- HgH
2 → Hg + H2
Biochemistry
Alireza Shayesteh et al conjectured that bacteria, containing mercuric reductase, such as Escherichia coli, can in theory reduce soluble mercury compounds to volatile HgH2, which should have a transient existence in nature.
Production
Hydride generation
Mercury(II) hydride can be produced by hydride generation. In this process, mercury(II) and tetrahydroborate or tetrahydroaluminate ions react to produce mercury(II) hydride according to the following equation:
- Hg2+
+ 2 MH−
4 → HgH
2 + 2 MH
3 (M = B or Al)
Direct synthesis
Mercury(II) hydride can also be generated by direct synthesis from the elements in the gas phase or in cryogenic inert gas martices:[5]
- Hg → Hg*
- Hg* + H
2 → [HgH
2]* - [HgH
2]* → HgH
2
This requires excitation of the mercury atom to the 1P or 3P state, as atomic mercury in its ground-state does not insert into the dihydrogen bond.[5] Excitation is accomplished by means of an ultraviolet-laser,[2] or electric discharge.[5] The initial yield is high; however, due to the product being in an excited state, a significant amount dissociates rapidly into mercury(I) hydride, then back into the initial reagents:
- [HgH
2]* → HgH + H - HgH → Hg + H
- 2 H → H
2
This is the preferred method for matrix isolation research. Besides mercury(II) hydride, it also produces other mercury hydrides in lesser quantities, such as the mercury(I) hydrides (HgH and Hg2H2).
References
- ↑ Yin, Runsheng; Krabbenhoft, David; Bergquist, Bridget; Zheng, Wang; Lepak, Ryan; Hurley, James (2016). "Effects of mercury and thallium concentrations on high precision determination of mercury isotopic composition by Neptune Plus multiple collector inductively coupled plasma mass spectrometry". Journal of Analytical Atomic Spectrometry. 31 (10): 2060–2068. doi:10.1039/C6JA00107F.
- 1 2 Legay-Sommaire, N.; F. Legay (1993). "Photochemistry in Hg doped matrices. Infrared spectra of mercury hydrides: HgH2, HgD2, HHgD, HgD". Chemical Physics Letters. 207 (2-3): 123–128. doi:10.1016/0009-2614(93)87001-j. ISSN 0009-2614.
- 1 2 3 4 Wang, Xuefeng; Andrews, Lester (2005). "Mercury dihydride forms a covalent molecular solid". Physical Chemistry Chemical Physics. 7 (5): 750. doi:10.1039/b412373e. ISSN 1463-9076.
- ↑ Shayesteh, Alireza; Yu, Shanshan; Bernath, Peter F. (2005). "Infrared Emission Spectra and Equilibrium Structures of Gaseous HgH2and HgD2". The Journal of Physical Chemistry A. 109 (45): 10280–10286. doi:10.1021/jp0540205. ISSN 1089-5639.
- 1 2 3 4 5 6 Shayesteh, Alireza; Shanshan Yu; Peter F. Bernath (2005). "Gaseous HgH2, CdH2, and ZnH2". Chemistry: A European Journal. 11 (16): 4709–4712. doi:10.1002/chem.200500332. ISSN 0947-6539.