Brine

Brine is a solution of salt (usually sodium chloride) in water. In different contexts, brine may refer to salt solutions ranging from about 3.5% (a typical concentration of seawater, or the lower end of solutions used for brining foods) up to about 26% (a typical saturated solution, depending on temperature). Other levels of concentration are called by different names:
| Fresh water | Brackish water | Saline water | Brine |
|---|---|---|---|
| < 0.05% | 0.05–3% | 3–5% | > 5% |
It is held that 0 °F (−17.78 °C and 255.37 Kelvin) was initially set as the zero point in the Fahrenheit temperature scale, as it was the coldest possible temperature that Daniel G. Fahrenheit could reliably reproduce by freezing brine. By reference, seawater usually freezes at -2°C (271.15 K).[1]
Properties
| NaCl, wt% | Freezing point (°C) | Density[lower-alpha 1] (g/cm3) | Refractive index[lower-alpha 2] at 589 nm | Viscosity[lower-alpha 3] (cP ) |
|---|---|---|---|---|
| 0 | 0 | 0.99984 | 1.333 | 1.002 |
| 0.5 | −0.3 | 1.0018 | 1.3339 | 1.011 |
| 1 | −0.59 | 1.0053 | 1.3347 | 1.02 |
| 2 | −1.19 | 1.0125 | 1.3365 | 1.036 |
| 3 | −1.79 | 1.0196 | 1.3383 | 1.052 |
| 4 | −2.41 | 1.0268 | 1.34 | 1.068 |
| 5 | −3.05 | 1.034 | 1.3418 | 1.085 |
| 6 | −3.7 | 1.0413 | 1.3435 | 1.104 |
| 7 | −4.38 | 1.0486 | 1.3453 | 1.124 |
| 8 | −5.08 | 1.0559 | 1.347 | 1.145 |
| 9 | −5.81 | 1.0633 | 1.3488 | 1.168 |
| 10 | −6.56 | 1.0707 | 1.3505 | 1.193 |
| 12 | −8.18 | 1.0857 | 1.3541 | 1.25 |
| 14 | −9.94 | 1.1008 | 1.3576 | 1.317 |
| 16 | −11.89 | 1.1162 | 1.3612 | 1.388 |
| 18 | −14.04 | 1.1319 | 1.3648 | 1.463 |
| 20 | −16.46 | 1.1478 | 1.3684 | 1.557 |
| 22 | −19.18 | 1.164 | 1.3721 | 1.676 |
At 100 °C (373.15 K, 212 °F), saturated sodium chloride brine is about 28% salt by weight i.e. 39.12 g salt dissolves in 100 mL of water at 100 °C. At 0 °C (273.15 K, 32 °F), brine can only hold about 26% salt.[3]
The thermal conductivity of seawater (3.5% dissolved salt by weight) is 0.6 W/mK at 25 °C.[4] The thermal conductivity decreases with increasing salinity and increases with increasing temperature; these graphs and online calculations plot thermal conductivity for varying salinity and temperature:[5]
Density of brine at various concentrations and temperatures can be approximated with a linear equation:[6] Density (lb/ft3)= a3 - (a2*Temperature (F)) where the values of an are:
| Weight % | a2 | a3 |
|---|---|---|
| 5 | .043 | 72.60 |
| 10 | .039 | 73.72 |
| 15 | .035 | 74.86 |
| 20 | .032 | 76.21 |
| 25 | .030 | 77.85 |
Electrolysis of brine
About four percent of hydrogen gas produced worldwide is created by electrolysis. The majority of this hydrogen produced through electrolysis is a side product in the production of chlorine.
- 2 NaCl(aq) + 2 H2O(l) → 2 NaOH(aq) + H2(g) + Cl2(g)
Usages
Refrigerating fluid
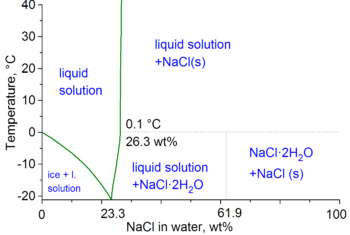
Brine is a common fluid used in large refrigeration installations for the transport of thermal energy from place to place. It is used because the addition of salt to water lowers the freezing temperature of the solution and the heat transport efficiency can be greatly enhanced for the comparatively low cost of the material. The lowest freezing point obtainable for NaCl brine is −21.1 °C (−6.0 °F) at 23.3wt% NaCl.[7] This is called the eutectic point.
De-icing
In lower temperatures, a brine solution can be used to de-ice or reduce freezing temperatures on roads.[8]
Culinary
In cooking, brine is used for food brining and salting.
Hydrology
In hydrology, brine is a form of salt water, namely, water with relatively high concentration of salt (usually sodium chloride).
See also
References
- ↑ gwydir.demon.co.uk
- ↑ Lide, D. R., ed. (2005). CRC Handbook of Chemistry and Physics (86th ed.). Boca Raton (FL): CRC Press. pp. 8–71, 8–116. ISBN 0-8493-0486-5.
- ↑ CRC Handbook of Chemistry and Physics, 63rd Edition 1982-1983.
- ↑ web.mit.edu
- ↑ twt.mpei.ac.ru
- ↑ Dittman, Gerald L. (February 16, 1977). "Calculation of Brine Properties.". Lawrence Livermore Laboratories. Livermore CA.
- ↑ webserver.dmt.upm.es Archived March 21, 2011, at the Wayback Machine.
- ↑ Iowa Department of Transportation