Causes of autism
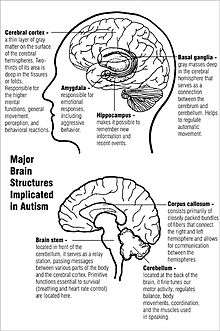
Many causes of autism have been proposed, but understanding of the theory of causation of autism and the other autism spectrum disorders (ASD) is incomplete.[1] Research indicates that genetic factors predominate. The heritability of autism, however, is complex, and it is typically unclear which genes are responsible.[2] In rare cases, autism is strongly associated with agents that cause birth defects.[3] Many other causes have been proposed, such as childhood immunizations, but numerous epidemiological studies have shown no scientific evidence supporting any link between vaccinations and autism.[4]
Related disorders
Autism involves atypical brain development which often becomes apparent in behavior and social development before a child is three years old. It can be characterized by impairments in social interaction and communication, as well as restricted interests and stereotyped behavior, and the characterization is independent of any underlying neurological defects.[5][6] Other characteristics include repetitive-like tasks seen in their behavior and sensory interests.[7] This article uses the terms autism and ASD to denote classical autism and the wider dispersion of symptoms and manifestations of autism, respectively.
Autism's theory of causation is incomplete.[1] It has long been presumed that there is a common cause at the genetic, cognitive, and neural levels for autism's characteristic triad of symptoms.[8] However, there is increasing suspicion among researchers that autism does not have a single cause, but is instead a complex disorder with a set of core aspects that have distinct causes.[8][9] Different underlying brain dysfunctions have been hypothesized to result in the common symptoms of autism, just as completely different brain problems result in intellectual disability. The terms autism or ASDs capture the wide range of disease processes at work.[10] Although these distinct causes have been hypothesized to often co-occur,[9] it has also been suggested that the correlation between the causes has been exaggerated.[11] The number of people known to have autism has increased dramatically since the 1980s, at least partly due to changes in diagnostic practice. It is unknown whether prevalence has increased as well.[12]
The consensus among mainstream autism researchers is that genetic factors predominate. Environmental factors that have been claimed to contribute to autism or exacerbate its symptoms, or that may be important to consider in future research, include certain foods,[13] infectious disease, heavy metals, solvents, diesel exhaust, PCBs, phthalates and phenols used in plastic products, pesticides, brominated flame retardants, alcohol, smoking, illicit drugs, and vaccines.[12] Among these factors, vaccines have attracted much attention, as parents may first become aware of autistic symptoms in their child around the time of a routine vaccination, and parental concern about vaccines has led to a decreasing uptake of childhood immunizations and an increasing likelihood of measles outbreaks. However, there is overwhelming scientific evidence showing no causal association between the measles-mumps-rubella (MMR) vaccine and autism, and there is no scientific evidence that the vaccine preservative thiomersal causes autism.[4][14]
Genetics
Genetic factors may be the most significant cause for autism spectrum disorders. Early studies of twins had estimated heritability to be over 90%, meaning that genetics explains over 90% of whether a child will develop autism.[15] However, this may be an overestimation, as new twin studies estimate the heritability at between 60-90%.[16][17] Many of the non-autistic co-twins had learning or social disabilities. For adult siblings the risk for having one or more features of the broader autism phenotype might be as high as 30%.[18]
However, in spite of the strong heritability, most cases of ASD occur sporadically with no recent evidence of family history. It has been hypothesized that spontaneous de novo mutations in the father's sperm or mother's egg contribute to the likelihood of developing autism.[19] There are two lines of evidence that support this hypothesis. Firstly, individuals with autism have significantly reduced fecundity, they are 20 times less likely to have children than average, thus curtailing the persistence of mutations in ASD genes over multiple generations in a family.[20] Secondly, the likelihood of having a child develop autism increases with advancing paternal age,[21] and mutations in sperm gradually accumulate throughout a man's life.[22]
The first genes to be definitively shown to contribute to risk for autism were found in the early 1990s by researchers looking at gender-specific forms of autism caused by mutations on the X chromosome. An expansion of the CGG trinucleotide repeat in the promoter of the gene FMR1 in boys causes fragile X syndrome, and at least 20% of boys with this mutation have behaviors consistent with autism spectrum disorder.[23] Mutations that inactivate the gene MECP2 cause Rett syndrome, which is associated with autistic behaviors in girls, and in boys the mutation is embryonic lethal.[24]
Besides these early examples, the role of de novo mutations in ASD first became evident when DNA microarray technologies reached sufficient resolution to allow the detection of copy number variation (CNV) in the human genome.[25][26] CNVs are the most common type of structural variation in the genome, consisting of deletions and duplications of DNA that range in size from a kilobase to a few megabases. Microarray analysis has shown that de novo CNVs occur at a significantly higher rate in sporadic cases of autism as compared to the rate in their typically developing siblings and unrelated controls. A series of studies have shown that gene disrupting de novo CNVs occur approximately four times more frequently in ASD than in controls and contribute to approximately 5-10% of cases.[19][27][28][29] Based on these studies, there are predicted to be 130-234 ASD-related CNV loci.[29] The first whole genome sequencing study to comprehensively catalog de novo structural variation at a much higher resolution than DNA microarray studies has shown that the mutation rate is approximately 20% and not elevated in autism compared to sibling controls.[30] However, structural variants in individuals with autism are much larger and four times more likely to disrupt genes, mirroring findings from CNV studies.[30]
CNV studies were closely followed by exome sequencing studies, which sequence the 1-2% of the genome that codes for proteins (the "exome"). These studies found that de novo gene inactivating mutations were observed in approximately 20% of individuals with autism, compared to 10% of unaffected siblings, suggesting the etiology of ASD is driven by these mutations in around 10% of cases.[31][32][33][34][35][36] There are predicted to be 350-450 genes that significantly increase susceptibility to ASDs when impacted by inactivating de novo mutations.[37] A further 12% of cases are predicted to be caused by protein altering missense mutations that change an amino acid but do not inactivate a gene.[33] Therefore approximately 30% of individuals with autism have a spontaneous de novo large CNV that deletes or duplicates genes, or mutation that changes the amino acid code of an individual gene. A further 5-10% of cases have inherited structural variation at loci known to be associated with autism, and these known structural variants may arise de novo in the parents of affected children.[30]
Tens of genes and CNVs have been definitively identified based on the observation of recurrent mutations in different individuals, and suggestive evidence has been found for over 100 others.[38] The Simons Foundation Autism Research Initiative (SFARI) details the evidence for each genetic locus associated with autism.[39]
These early gene and CNV findings have shown that the cognitive and behavioral features associated with each of the underlying mutations is variable. Each mutation is itself associated with a variety of clinical diagnoses, and can also be found in a small percentage of individuals with no clinical diagnosis.[40][41] Thus the genetic disorders that comprise autism are not autism-specific. The mutations themselves are characterized by considerable variability in clinical outcome and typically only a subset of mutation carriers meet criteria for autism. This variable expressivity results in different individuals with the same mutation varying considerably in the severity of their observed particular trait.[42]
The conclusion of these recent studies of de novo mutation is that the spectrum of autism is breaking up into quanta of individual disorders defined by genetics.[42]
Epigenetics
Epigenetic mechanisms may increase the risk of autism. Epigenetic changes occur as a result not of DNA sequence changes but of chromosomal histone modification or modification of the DNA bases. Such modifications are known to be affected by environmental factors, including nutrition, drugs, and mental stress.[43] Interest has been expressed in imprinted regions on chromosomes 15q and 7q.[44]
Prenatal environment
The risk of autism is associated with several prenatal risk factors, including advanced age in either parent, diabetes, bleeding, and use of psychiatric drugs in the mother during pregnancy.[45] Autism has been linked to birth defect agents acting during the first eight weeks from conception, though these cases are rare.[46]
Infectious processes
Prenatal viral infection has been called the principal non-genetic cause of autism. Prenatal exposure to rubella or cytomegalovirus activates the mother's immune response and greatly increases the risk for autism.[47] Congenital rubella syndrome is the most convincing environmental cause of autism.[48] Infection-associated immunological events in early pregnancy may affect neural development more than infections in late pregnancy, not only for autism, but also for psychiatric disorders of presumed neurodevelopmental origin, notably schizophrenia.[49]
Environmental agents
Teratogens are environmental agents that cause birth defects. Some agents that are theorized to cause birth defects have also been suggested as potential autism risk factors, although there is little to no scientific evidence to back such claims. These include exposure of the embryo to valproic acid,[50] thalidomide or misoprostol.[51] These cases are rare.[52] Questions have also been raised whether ethanol (grain alcohol) increases autism risk, as part of fetal alcohol syndrome or alcohol-related birth defects.[51] All known teratogens appear to act during the first eight weeks from conception, and though this does not exclude the possibility that autism can be initiated or affected later, it is strong evidence that autism arises very early in development.[3]
Other maternal conditions
Thyroid problems that lead to thyroxine deficiency in the mother in weeks 8–12 of pregnancy have been postulated to produce changes in the fetal brain leading to autism. Thyroxine deficiencies can be caused by inadequate iodine in the diet, and by environmental agents that interfere with iodine uptake or act against thyroid hormones. Possible environmental agents include flavonoids in food, tobacco smoke, and most herbicides. This hypothesis has not been tested.[53]
Diabetes in the mother during pregnancy is a significant risk factor for autism; a 2009 meta-analysis found that gestational diabetes was associated with a twofold increased risk. A 2014 review also found that maternal diabetes was significantly associated with an increased risk of ASD.[54] Although diabetes causes metabolic and hormonal abnormalities and oxidative stress, no biological mechanism is known for the association between gestational diabetes and autism risk.[45]
Maternal obesity during pregnancy may also increase the risk of autism, although further study is needed.[55]
Other in utero
It has been hypothesized that folic acid taken during pregnancy could play a role in reducing cases of autism by modulating gene expression through an epigenetic mechanism. This hypothesis is supported by multiple studies.[56]
Prenatal stress, consisting of exposure to life events or environmental factors that distress an expectant mother, has been hypothesized to contribute to autism, possibly as part of a gene-environment interaction. Autism has been reported to be associated with prenatal stress both with retrospective studies that examined stressors such as job loss and family discord, and with natural experiments involving prenatal exposure to storms; animal studies have reported that prenatal stress can disrupt brain development and produce behaviors resembling symptoms of autism.[57]
The fetal testosterone theory hypothesizes that higher levels of testosterone in the amniotic fluid of mothers pushes brain development towards improved ability to see patterns and analyze complex systems while diminishing communication and empathy, emphasizing "male" traits over "female", or in E-S theory terminology, emphasizing "systemizing" over "empathizing". One project has published several reports suggesting that high levels of fetal testosterone could produce behaviors relevant to those seen in autism.[58]
Based in part on animal studies, diagnostic ultrasounds administered during pregnancy have been hypothesized to increase the child's risk of autism. This hypothesis is not supported by independently published research, and examination of children whose mothers received an ultrasound has failed to find evidence of harmful effects.[59]
Some research suggests that maternal exposure to selective serotonin reuptake inhibitors during pregnancy is associated with an increased risk of autism, but it remains unclear whether there is a causal link between the two.[60]
Perinatal environment
Autism is associated with some perinatal and obstetric conditions. A 2007 review of risk factors found associated obstetric conditions that included low birth weight and gestation duration, and hypoxia during childbirth. This association does not demonstrate a causal relationship. As a result, an underlying cause could explain both autism and these associated conditions.[61] There is growing evidence that perinatal exposure to air pollution may be a risk factor for autism,[62] although this evidence suffers from methodological limitations, including a small number of studies and failure to control for potential confounding factors.[63]
Postnatal environment
A wide variety of postnatal contributors to autism have been proposed, including gastrointestinal or immune system abnormalities, allergies, and exposure of children to drugs, vaccines, infection, certain foods, or heavy metals. The evidence for these risk factors is anecdotal and has not been confirmed by reliable studies.[64]
Amygdala neurons
This theory hypothesizes that an early developmental failure involving the amygdala cascades on the development of cortical areas that mediate social perception in the visual domain. The fusiform face area of the ventral stream is implicated. The idea is that it is involved in social knowledge and social cognition, and that the deficits in this network are instrumental in causing autism.[65]
Autoimmune disease
This theory hypothesizes that autoantibodies that target the brain or elements of brain metabolism may cause or exacerbate autism. It is related to the maternal infection theory, except that it postulates that the effect is caused by the individual's own antibodies, possibly due to an environmental trigger after birth. It is also related to several other hypothesized causes; for example, viral infection has been hypothesized to cause autism via an autoimmune mechanism.[66]
Interactions between the immune system and the nervous system begin early during embryogenesis, and successful neurodevelopment depends on a balanced immune response. It is possible that aberrant immune activity during critical periods of neurodevelopment is part of the mechanism of some forms of ASD.[67] A small percentage of autism cases are associated with infection, usually before birth. Results from immune studies have been contradictory. Some abnormalities have been found in specific subgroups, and some of these have been replicated. It is not known whether these abnormalities are relevant to the pathology of autism, for example, by infection or autoimmunity, or whether they are secondary to the disease processes.[68] As autoantibodies are found in diseases other than ASD, and are not always present in ASD,[69] the relationship between immune disturbances and autism remains unclear and controversial.[70] A 2015 systematic review and meta-analysis found that children with a family history of autoimmune diseases were at a greater risk of autism compared to children without such a history.[71]
When an underlying maternal autoimmune disease is present, antibodies circulating to the fetus could contribute to the development of autism spectrum disorders.[72]
Endogenous opiate precursor theory
In 1979, Jaak Panksepp proposed a connection between autism and opiates, noting that injections of minute quantities of opiates in young laboratory animals induce symptoms similar to those observed among autistic children.[73] Opiate theory hypothesizes that autism is caused by a digestive disorder present from birth which causes gluten (present in wheat-derived foods) and casein (present in dairy products) to be converted to the opioid peptides gliadorphin (aka gluteomorphin) and casomorphin.
According to the theory, exposure to these opiate compounds in young children interferes with normal neurological development by dulling sensory input. Lacking sufficient sensory input, the developing brain attempts to artificially generate the auditory, vestibular, visual, and tactile input on its own. This attempt at generating input manifests itself as behaviors common to autism, such as grunting or screaming (auditory), spinning or rocking back and forth (vestibular), preoccupation with spinning objects or waving of the fingers in front of the eyes (visual), and hand flapping or self-injury (tactile).
The theory further states that removing opiate precursors from a child's diet may allow time for these behaviors to cease, and neurological development in very young children to resume normally.[74] The possibility of a relationship between autism and the consumption of gluten and casein was first articulated by Kalle Reichelt in 1991.[75] The scientific evidence is not yet adequate to make treatment recommendations regarding diets, such as the GFCF diet, which exclude these substances.[76]
Gastrointestinal connection
Parents have reported gastrointestinal (GI) disturbances in autistic children, and several studies have investigated possible associations between autism and the gut,[77] but the results so far are inconclusive.
There is some research evidence that autistic children are more likely to have GI symptoms than typical children.[78] Even so, design flaws in studies of elimination diets mean that the data are inadequate to guide treatment recommendations.[13]
After a preliminary 1998 study of three children with ASD treated with secretin infusion reported improved GI function and dramatic improvement in behavior, many parents sought secretin treatment and a black market for the hormone developed quickly.[77] Later studies found secretin clearly ineffective in treating autism.[79]
Lack of vitamin D
There is limited evidence for the hypothesis that vitamin D deficiency has a role in autism, and it may be biologically plausible,[80] but more research is needed.[81]
Lead
Lead poisoning has been suggested as a possible risk factor for autism, as the lead blood levels of autistic children has been reported to be significantly higher than typical.[82] The atypical eating behaviors of autistic children, along with habitual mouthing and pica, make it hard to determine whether increased lead levels are a cause or a consequence of autism.[82]
Locus coeruleus–noradrenergic system
This theory hypothesizes that autistic behaviors depend at least in part on a developmental dysregulation that results in impaired function of the locus coeruleus–noradrenergic (LC-NA) system. The LC-NA system is heavily involved in arousal and attention; for example, it is related to the brain's acquisition and use of environmental cues.[83]
Mercury
This theory hypothesizes that autism is associated with mercury poisoning, based on perceived similarity of symptoms and reports of mercury or its biomarkers in some autistic children.[84] This view has gained little traction in the scientific community as the typical symptoms of mercury toxicity are significantly different from symptoms seen in autism.[85] The principal source of human exposure to organic mercury is via fish consumption and for inorganic mercury is dental amalgams. Other forms of exposure, such as in cosmetics and vaccines, also occur. The evidence so far is indirect for the association between autism and mercury exposure after birth, as no direct test has been reported, and there is no evidence of an association between autism and postnatal exposure to any neurotoxicant.[86] A meta-analysis published in 2007 concluded that there was no link between mercury and autism.[87]
Oxidative stress
This theory hypothesizes that toxicity and oxidative stress may cause autism in some cases. Evidence includes genetic effects on metabolic pathways, reduced antioxidant capacity, enzyme changes, and enhanced biomarkers for oxidative stress; however, the overall evidence is weaker than it is for involvement oxidative stress with disorders such as schizophrenia.[88] One theory is that stress damages Purkinje cells in the cerebellum after birth, and it is possible that glutathione is involved.[89] Autistic children have lower levels of total glutathione, and higher levels of oxidized glutathione.[90] Based on this theory, antioxidants may be a useful treatment for autism.[91]
Refrigerator mother
Child psychologist Bruno Bettelheim believed that autism was linked to early childhood trauma, and his work was highly influential for decades both in the medical and popular spheres. Parents, especially mothers, of individuals with autism were blamed for having caused their child's condition through the withholding of affection.[92] Leo Kanner, who first described autism,[93] suggested that parental coldness might contribute to autism.[94] Although Kanner eventually renounced the theory, Bettelheim put an almost exclusive emphasis on it in both his medical and his popular books. Treatments based on these theories failed to help children with autism, and after Bettelheim's death, it came out that his reported rates of cure (around 85%) were found to be fraudulent.[95]
Vaccines
Scientific studies have refuted a causal relationship between vaccinations and autism.[96][97][98] Despite this, some parents believe that vaccinations cause autism and therefore delay or avoid immunizing their children under the "vaccine overload" hypothesis that giving many vaccines at once may overwhelm a child's immune system and lead to autism,[99] even though this hypothesis has no scientific evidence and is biologically implausible.[100] Because diseases such as measles can cause severe disabilities and death, the risk of death or disability for an unvaccinated child is higher than the risk for a child who has been vaccinated.[101]
MMR vaccine
The MMR vaccine hypothesis of autism is one of the most extensively debated hypothesies regarding the origins of autism. Andrew Wakefield et al. reported a study of 12 children who had autism and bowel symptoms, in some cases reportedly with onset after MMR.[102] Although the paper, which was later retracted by the journal,[102] concluded "We did not prove an association between measles, mumps, and rubella vaccine and the syndrome described,"[103] Wakefield nevertheless suggested during a 1998 press conference that giving children the vaccines in three separate doses would be safer than a single dose.
In 2004, the interpretation of a causal link between MMR vaccine and autism was formally retracted by ten of Wakefield's twelve co-authors.[104] The retraction followed an investigation by The Sunday Times, which stated that Wakefield "acted dishonestly and irresponsibly".[105] The Centers for Disease Control and Prevention,[106] the Institute of Medicine of the National Academy of Sciences,[107] and the U.K. National Health Service[108] have all concluded that there is no evidence of a link between the MMR vaccine and autism.
In February 2010, The Lancet, which published Wakefield's study, fully retracted it after an independent auditor found the study to be flawed.[102] In January 2011, an investigation published in the journal BMJ described the Wakefield study as the result of deliberate fraud and manipulation of data.[109][110][111][112]
Thiomersal (thimerosal)
Perhaps the best-known hypothesis involving mercury and autism involves the use of the mercury-based compound thiomersal, a preservative that has been phased out from most childhood vaccinations in developed countries including US and the EU.[113] Parents may first become aware of autistic symptoms in their child around the time of a routine vaccination. There is no scientific evidence for a causal connection between thiomersal and autism, but parental concern about the thiomersal controversy has led to decreasing rates of childhood immunizations[4] and increasing likelihood of disease outbreaks.[114][115] Because of public concerns, thiomersal content was completely removed or dramatically reduced from childhood vaccines that contained it in the 1990s; despite this, autism rates continued to climb well into the late 2000s.
A causal link between thimerosal and autism has been rejected by international scientific and medical professional bodies including the American Medical Association,[116] the American Academy of Pediatrics,[117] the American College of Medical Toxicology,[118] the Canadian Paediatric Society,[119] the U.S. National Academy of Sciences,[107] the Food and Drug Administration,[120] Centers for Disease Control and Prevention,[106] the World Health Organization,[121] the Public Health Agency of Canada,[122] and the European Medicines Agency.[123]
Viral infection
Many studies have presented evidence for and against association of autism with viral infection after birth. Laboratory rats infected with Borna disease virus show some symptoms similar to those of autism but blood studies of autistic children show no evidence of infection by this virus. Members of the herpes virus family may have a role in autism, but the evidence so far is anecdotal. Viruses have long been suspected as triggers for immune-mediated diseases such as multiple sclerosis but showing a direct role for viral causation is difficult in those diseases, and mechanisms whereby viral infections could lead to autism are speculative.[47]
Social construct
The social construct theory says that the boundary between normal and abnormal is subjective and arbitrary, so autism does not exist as an objective entity, but only as a social construct. It further argues that autistic individuals themselves have a way of being that is partly socially constructed.[124]
Asperger syndrome and high-functioning autism are particular targets of the theory that social factors determine what it means to be autistic. The theory hypothesizes that individuals with these diagnoses inhabit the identities that have been ascribed to them, and promote their sense of well-being by resisting or appropriating autistic ascriptions.[125]
See also
References
- 1 2 Trottier G, Srivastava L, Walker CD. Etiology of infantile autism: a review of recent advances in genetic and neurobiological research. J Psychiatry Neurosci. 1999;24(2):103–115. PMID 10212552.
- ↑ Freitag CM. The genetics of autistic disorders and its clinical relevance: a review of the literature. Mol Psychiatry. 2007;12(1):2–22. doi:10.1038/sj.mp.4001896. PMID 17033636.
- 1 2 Arndt TL, Stodgell CJ, Rodier PM. The teratology of autism. Int J Dev Neurosci. 2005;23(2–3):189–99. doi:10.1016/j.ijdevneu.2004.11.001. PMID 15749245.
- 1 2 3 Doja A, Roberts W. Immunizations and autism: a review of the literature. Can J Neurol Sci. 2006;33(4):341–6. doi:10.1017/s031716710000528x. PMID 17168158.
- ↑ American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th, text revision (DSM-IV-TR) ed. 2000 [Retrieved 2009-02-17]. ISBN 0-89042-025-4. Diagnostic criteria for 299.00 Autistic Disorder.
- ↑ World Health Organization. International Statistical Classification of Diseases and Related Health Problems. 10th (ICD-10) ed. 2006 [Retrieved 2007-06-25]. F84. Pervasive developmental disorders.
- ↑ McPartland, James C.; Law, Karen; Dawson, Geraldine (August 26, 2015). "Autism Spectrum Disorder". Encyclopedia of Mental Health (Second Edition): 124–130. doi:10.1016/B978-0-12-397045-9.00230-5.
- 1 2 Happé F, Ronald A. The 'fractionable autism triad': a review of evidence from behavioural, genetic, cognitive and neural research. Neuropsychol Rev. 2008;18(4):287–304. doi:10.1007/s11065-008-9076-8. PMID 18956240.
- 1 2 Happé F, Ronald A, Plomin R. Time to give up on a single explanation for autism. Nat Neurosci. 2006;9(10):1218–20. doi:10.1038/nn1770. PMID 17001340.
- ↑ Geschwind DH. Advances in autism. Annu Rev Med. 2009;60:367–80. doi:10.1146/annurev.med.60.053107.121225. PMID 19630577.
- ↑ Mandy WP, Skuse DH. What is the association between the social-communication element of autism and repetitive interests, behaviours and activities? J Child Psychol Psychiatry. 2008;49(8):795–808. doi:10.1111/j.1469-7610.2008.01911.x. PMID 18564070.
- 1 2 Newschaffer CJ, Croen LA, Daniels J et al.. The epidemiology of autism spectrum disorders [PDF]. Annu Rev Public Health. 2007 [Retrieved 2009-10-10];28:235–58. doi:10.1146/annurev.publhealth.28.021406.144007. PMID 17367287.
- 1 2 Christison GW, Ivany K. Elimination diets in autism spectrum disorders: any wheat amidst the chaff? J Dev Behav Pediatr. 2006;27(2 Suppl 2):S162–71. doi:10.1097/00004703-200604002-00015. PMID 16685183.
- ↑ Taylor B. Vaccines and the changing epidemiology of autism. Child Care Health Dev. 2006;32(5):511–9. doi:10.1111/j.1365-2214.2006.00655.x. PMID 16919130.
- ↑ Freitag, C M (10 October 2006). "The genetics of autistic disorders and its clinical relevance: a review of the literature". Molecular Psychiatry. 12 (1): 2–22. doi:10.1038/sj.mp.4001896. PMID 17033636.
- ↑ Hallmayer, Joachim (1 November 2011). "Genetic Heritability and Shared Environmental Factors Among Twin Pairs With Autism". Archives of General Psychiatry. 68 (11): 1095. doi:10.1001/archgenpsychiatry.2011.76.
- ↑ Ronald, Angelica; Hoekstra, Rosa A. (April 2011). "Autism spectrum disorders and autistic traits: A decade of new twin studies". American Journal of Medical Genetics Part B. 156 (3): 255–274. doi:10.1002/ajmg.b.31159.
- ↑ Folstein SE, Rosen-Sheidley B. Genetics of autism: complex aetiology for a heterogeneous disorder. Nat Rev Genet. 2001;2(12):943–55. doi:10.1038/35103559. PMID 11733747.
- 1 2 Sebat, J.; Lakshmi, B.; Malhotra, D.; Troge, J.; Lese-Martin, C.; Walsh, T.; Yamrom, B.; Yoon, S.; Krasnitz, A.; Kendall, J.; Leotta, A.; Pai, D.; Zhang, R.; Lee, Y.-H.; Hicks, J.; Spence, S. J.; Lee, A. T.; Puura, K.; Lehtimaki, T.; Ledbetter, D.; Gregersen, P. K.; Bregman, J.; Sutcliffe, J. S.; Jobanputra, V.; Chung, W.; Warburton, D.; King, M.-C.; Skuse, D.; Geschwind, D. H.; Gilliam, T. C.; Ye, K.; Wigler, M. (20 April 2007). "Strong Association of De Novo Copy Number Mutations with Autism". Science. 316 (5823): 445–449. doi:10.1126/science.1138659. PMC 2993504
 . PMID 17363630.
. PMID 17363630. - ↑ Uher, R (25 August 2009). "The role of genetic variation in the causation of mental illness: an evolution-informed framework". Molecular Psychiatry. 14 (12): 1072–1082. doi:10.1038/mp.2009.85.
- ↑ Hultman, C M; Sandin, S; Levine, S Z; Lichtenstein, P; Reichenberg, A (30 November 2010). "Advancing paternal age and risk of autism: new evidence from a population-based study and a meta-analysis of epidemiological studies". Molecular Psychiatry. 16 (12): 1203–1212. doi:10.1038/mp.2010.121.
- ↑ Kong, Augustine; Frigge, Michael L.; Masson, Gisli; Besenbacher, Soren; Sulem, Patrick; Magnusson, Gisli; Gudjonsson, Sigurjon A.; Sigurdsson, Asgeir; Jonasdottir, Aslaug; Jonasdottir, Adalbjorg; Wong, Wendy S. W.; Sigurdsson, Gunnar; Walters, G. Bragi; Steinberg, Stacy; Helgason, Hannes; Thorleifsson, Gudmar; Gudbjartsson, Daniel F.; Helgason, Agnar; Magnusson, Olafur Th.; Thorsteinsdottir, Unnur; Stefansson, Kari (22 August 2012). "Rate of de novo mutations and the importance of father's age to disease risk". Nature. 488 (7412): 471–475. doi:10.1038/nature11396. PMC 3548427
 . PMID 22914163.
. PMID 22914163. - ↑ Hatton, Deborah D.; Sideris, John; Skinner, Martie; Mankowski, Jean; Bailey, Donald B.; Roberts, Jane; Mirrett, Penny (1 September 2006). "Autistic behavior in children with fragile X syndrome: Prevalence, stability, and the impact of FMRP". American Journal of Medical Genetics Part A. 140A (17): 1804–1813. doi:10.1002/ajmg.a.31286.
- ↑ Zoghbi, Huda Y.; Amir, Ruthie E.; Van den Veyver, Ignatia B.; Wan, Mimi; Tran, Charles Q.; Francke, Uta (1 October 1999). "Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2". Nature Genetics. 23 (2): 185–188. doi:10.1038/13810. PMID 10508514.
- ↑ Sebat, J. (23 July 2004). "Large-Scale Copy Number Polymorphism in the Human Genome". Science. 305 (5683): 525–528. doi:10.1126/science.1098918. PMID 15273396.
- ↑ Iafrate, A John; Feuk, Lars; Rivera, Miguel N; Listewnik, Marc L; Donahoe, Patricia K; Qi, Ying; Scherer, Stephen W; Lee, Charles (1 August 2004). "Detection of large-scale variation in the human genome". Nature Genetics. 36 (9): 949–951. doi:10.1038/ng1416. PMID 15286789.
- ↑ Pinto, Dalila; Delaby, Elsa; Merico, Daniele; Barbosa, Mafalda; Merikangas, Alison; Klei, Lambertus; Thiruvahindrapuram, Bhooma; Xu, Xiao; Ziman, Robert; Wang, Zhuozhi; Vorstman, Jacob A.S.; Thompson, Ann; Regan, Regina; Pilorge, Marion; Pellecchia, Giovanna; Pagnamenta, Alistair T.; Oliveira, Bárbara; Marshall, Christian R.; Magalhaes, Tiago R.; Lowe, Jennifer K.; Howe, Jennifer L.; Griswold, Anthony J.; Gilbert, John; Duketis, Eftichia; Dombroski, Beth A.; De Jonge, Maretha V.; Cuccaro, Michael; Crawford, Emily L.; Correia, Catarina T.; Conroy, Judith; Conceição, Inês C.; Chiocchetti, Andreas G.; Casey, Jillian P.; Cai, Guiqing; Cabrol, Christelle; Bolshakova, Nadia; Bacchelli, Elena; Anney, Richard; Gallinger, Steven; Cotterchio, Michelle; Casey, Graham; Zwaigenbaum, Lonnie; Wittemeyer, Kerstin; Wing, Kirsty; Wallace, Simon; van Engeland, Herman; Tryfon, Ana; Thomson, Susanne; Soorya, Latha; Rogé, Bernadette; Roberts, Wendy; Poustka, Fritz; Mouga, Susana; Minshew, Nancy; McInnes, L. Alison; McGrew, Susan G.; Lord, Catherine; Leboyer, Marion; Le Couteur, Ann S.; Kolevzon, Alexander; Jiménez González, Patricia; Jacob, Suma; Holt, Richard; Guter, Stephen; Green, Jonathan; Green, Andrew; Gillberg, Christopher; Fernandez, Bridget A.; Duque, Frederico; Delorme, Richard; Dawson, Geraldine; Chaste, Pauline; Café, Cátia; Brennan, Sean; Bourgeron, Thomas; Bolton, Patrick F.; Bölte, Sven; Bernier, Raphael; Baird, Gillian; Bailey, Anthony J.; Anagnostou, Evdokia; Almeida, Joana; Wijsman, Ellen M.; Vieland, Veronica J.; Vicente, Astrid M.; Schellenberg, Gerard D.; Pericak-Vance, Margaret; Paterson, Andrew D.; Parr, Jeremy R.; Oliveira, Guiomar; Nurnberger, John I.; Monaco, Anthony P.; Maestrini, Elena; Klauck, Sabine M.; Hakonarson, Hakon; Haines, Jonathan L.; Geschwind, Daniel H.; Freitag, Christine M.; Folstein, Susan E.; Ennis, Sean; Coon, Hilary; Battaglia, Agatino; Szatmari, Peter; Sutcliffe, James S.; Hallmayer, Joachim; Gill, Michael; Cook, Edwin H.; Buxbaum, Joseph D.; Devlin, Bernie; Gallagher, Louise; Betancur, Catalina; Scherer, Stephen W. (May 2014). "Convergence of Genes and Cellular Pathways Dysregulated in Autism Spectrum Disorders". The American Journal of Human Genetics. 94 (5): 677–694. doi:10.1016/j.ajhg.2014.03.018.
- ↑ Levy, Dan; Ronemus, Michael; Yamrom, Boris; Lee, Yoon-ha; Leotta, Anthony; Kendall, Jude; Marks, Steven; Lakshmi, B.; Pai, Deepa; Ye, Kenny; Buja, Andreas; Krieger, Abba; Yoon, Seungtai; Troge, Jennifer; Rodgers, Linda; Iossifov, Ivan; Wigler, Michael (June 2011). "Rare De Novo and Transmitted Copy-Number Variation in Autistic Spectrum Disorders". Neuron. 70 (5): 886–897. doi:10.1016/j.neuron.2011.05.015.
- 1 2 Sanders, Stephan J.; Ercan-Sencicek, A. Gulhan; Hus, Vanessa; Luo, Rui; Murtha, Michael T.; Moreno-De-Luca, Daniel; Chu, Su H.; Moreau, Michael P.; Gupta, Abha R.; Thomson, Susanne A.; Mason, Christopher E.; Bilguvar, Kaya; Celestino-Soper, Patricia B.S.; Choi, Murim; Crawford, Emily L.; Davis, Lea; Davis Wright, Nicole R.; Dhodapkar, Rahul M.; DiCola, Michael; DiLullo, Nicholas M.; Fernandez, Thomas V.; Fielding-Singh, Vikram; Fishman, Daniel O.; Frahm, Stephanie; Garagaloyan, Rouben; Goh, Gerald S.; Kammela, Sindhuja; Klei, Lambertus; Lowe, Jennifer K.; Lund, Sabata C.; McGrew, Anna D.; Meyer, Kyle A.; Moffat, William J.; Murdoch, John D.; O'Roak, Brian J.; Ober, Gordon T.; Pottenger, Rebecca S.; Raubeson, Melanie J.; Song, Youeun; Wang, Qi; Yaspan, Brian L.; Yu, Timothy W.; Yurkiewicz, Ilana R.; Beaudet, Arthur L.; Cantor, Rita M.; Curland, Martin; Grice, Dorothy E.; Günel, Murat; Lifton, Richard P.; Mane, Shrikant M.; Martin, Donna M.; Shaw, Chad A.; Sheldon, Michael; Tischfield, Jay A.; Walsh, Christopher A.; Morrow, Eric M.; Ledbetter, David H.; Fombonne, Eric; Lord, Catherine; Martin, Christa Lese; Brooks, Andrew I.; Sutcliffe, James S.; Cook, Edwin H.; Geschwind, Daniel; Roeder, Kathryn; Devlin, Bernie; State, Matthew W. (June 2011). "Multiple Recurrent De Novo CNVs, Including Duplications of the 7q11.23 Williams Syndrome Region, Are Strongly Associated with Autism". Neuron. 70 (5): 863–885. doi:10.1016/j.neuron.2011.05.002.
- 1 2 3 Brandler, William M.; Antaki, Danny; Gujral, Madhusudan; Noor, Amina; Rosanio, Gabriel; Chapman, Timothy R.; Barrera, Daniel J.; Lin, Guan Ning; Malhotra, Dheeraj; Watts, Amanda C.; Wong, Lawrence C.; Estabillo, Jasper A.; Gadomski, Therese E.; Hong, Oanh; Fajardo, Karin V. Fuentes; Bhandari, Abhishek; Owen, Renius; Baughn, Michael; Yuan, Jeffrey; Solomon, Terry; Moyzis, Alexandra G.; Maile, Michelle S.; Sanders, Stephan J.; Reiner, Gail E.; Vaux, Keith K.; Strom, Charles M.; Zhang, Kang; Muotri, Alysson R.; Akshoomoff, Natacha; Leal, Suzanne M.; Pierce, Karen; Courchesne, Eric; Iakoucheva, Lilia M.; Corsello, Christina; Sebat, Jonathan (March 2016). "Frequency and Complexity of De Novo Structural Mutation in Autism". The American Journal of Human Genetics. 98 (4): 1–13. doi:10.1016/j.ajhg.2016.02.018.
- ↑ Iossifov, Ivan; Ronemus, Michael; Levy, Dan; Wang, Zihua; Hakker, Inessa; Rosenbaum, Julie; Yamrom, Boris; Lee, Yoon-ha; Narzisi, Giuseppe; Leotta, Anthony; Kendall, Jude; Grabowska, Ewa; Ma, Beicong; Marks, Steven; Rodgers, Linda; Stepansky, Asya; Troge, Jennifer; Andrews, Peter; Bekritsky, Mitchell; Pradhan, Kith; Ghiban, Elena; Kramer, Melissa; Parla, Jennifer; Demeter, Ryan; Fulton, Lucinda L.; Fulton, Robert S.; Magrini, Vincent J.; Ye, Kenny; Darnell, Jennifer C.; Darnell, Robert B.; Mardis, Elaine R.; Wilson, Richard K.; Schatz, Michael C.; McCombie, W. Richard; Wigler, Michael (April 2012). "De Novo Gene Disruptions in Children on the Autistic Spectrum". Neuron. 74 (2): 285–299. doi:10.1016/j.neuron.2012.04.009.
- ↑ De Rubeis, Silvia; He, Xin; Goldberg, Arthur P.; Poultney, Christopher S.; Samocha, Kaitlin; Ercument Cicek, A.; Kou, Yan; Liu, Li; Fromer, Menachem; Walker, Susan; Singh, Tarjinder; Klei, Lambertus; Kosmicki, Jack; Fu, Shih-Chen; Aleksic, Branko; Biscaldi, Monica; Bolton, Patrick F.; Brownfeld, Jessica M.; Cai, Jinlu; Campbell, Nicholas G.; Carracedo, Angel; Chahrour, Maria H.; Chiocchetti, Andreas G.; Coon, Hilary; Crawford, Emily L.; Crooks, Lucy; Curran, Sarah R.; Dawson, Geraldine; Duketis, Eftichia; Fernandez, Bridget A.; Gallagher, Louise; Geller, Evan; Guter, Stephen J.; Sean Hill, R.; Ionita-Laza, Iuliana; Jimenez Gonzalez, Patricia; Kilpinen, Helena; Klauck, Sabine M.; Kolevzon, Alexander; Lee, Irene; Lei, Jing; Lehtimäki, Terho; Lin, Chiao-Feng; Ma’ayan, Avi; Marshall, Christian R.; McInnes, Alison L.; Neale, Benjamin; Owen, Michael J.; Ozaki, Norio; Parellada, Mara; Parr, Jeremy R.; Purcell, Shaun; Puura, Kaija; Rajagopalan, Deepthi; Rehnström, Karola; Reichenberg, Abraham; Sabo, Aniko; Sachse, Michael; Sanders, Stephan J.; Schafer, Chad; Schulte-Rüther, Martin; Skuse, David; Stevens, Christine; Szatmari, Peter; Tammimies, Kristiina; Valladares, Otto; Voran, Annette; Wang, Li-San; Weiss, Lauren A.; Jeremy Willsey, A.; Yu, Timothy W.; Yuen, Ryan K. C.; Cook, Edwin H.; Freitag, Christine M.; Gill, Michael; Hultman, Christina M.; Lehner, Thomas; Palotie, Aarno; Schellenberg, Gerard D.; Sklar, Pamela; State, Matthew W.; Sutcliffe, James S.; Walsh, Christopher A.; Scherer, Stephen W.; Zwick, Michael E.; Barrett, Jeffrey C.; Cutler, David J.; Roeder, Kathryn; Devlin, Bernie; Daly, Mark J.; Buxbaum, Joseph D. (29 October 2014). "Synaptic, transcriptional and chromatin genes disrupted in autism". Nature. 515 (7526): 209–215. doi:10.1038/nature13772. PMC 4402723
 . PMID 25363760.
. PMID 25363760. - 1 2 Iossifov, Ivan; O’Roak, Brian J.; Sanders, Stephan J.; Ronemus, Michael; Krumm, Niklas; Levy, Dan; Stessman, Holly A.; Witherspoon, Kali T.; Vives, Laura; Patterson, Karynne E.; Smith, Joshua D.; Paeper, Bryan; Nickerson, Deborah A.; Dea, Jeanselle; Dong, Shan; Gonzalez, Luis E.; Mandell, Jeffrey D.; Mane, Shrikant M.; Murtha, Michael T.; Sullivan, Catherine A.; Walker, Michael F.; Waqar, Zainulabedin; Wei, Liping; Willsey, A. Jeremy; Yamrom, Boris; Lee, Yoon-ha; Grabowska, Ewa; Dalkic, Ertugrul; Wang, Zihua; Marks, Steven; Andrews, Peter; Leotta, Anthony; Kendall, Jude; Hakker, Inessa; Rosenbaum, Julie; Ma, Beicong; Rodgers, Linda; Troge, Jennifer; Narzisi, Giuseppe; Yoon, Seungtai; Schatz, Michael C.; Ye, Kenny; McCombie, W. Richard; Shendure, Jay; Eichler, Evan E.; State, Matthew W.; Wigler, Michael (29 October 2014). "The contribution of de novo coding mutations to autism spectrum disorder". Nature. 515 (7526): 216–221. doi:10.1038/nature13908. PMID 25363768.
- ↑ Neale, Benjamin M.; Kou, Yan; Liu, Li; Ma’ayan, Avi; Samocha, Kaitlin E.; Sabo, Aniko; Lin, Chiao-Feng; Stevens, Christine; Wang, Li-San; Makarov, Vladimir; Polak, Paz; Yoon, Seungtai; Maguire, Jared; Crawford, Emily L.; Campbell, Nicholas G.; Geller, Evan T.; Valladares, Otto; Schafer, Chad; Liu, Han; Zhao, Tuo; Cai, Guiqing; Lihm, Jayon; Dannenfelser, Ruth; Jabado, Omar; Peralta, Zuleyma; Nagaswamy, Uma; Muzny, Donna; Reid, Jeffrey G.; Newsham, Irene; Wu, Yuanqing; Lewis, Lora; Han, Yi; Voight, Benjamin F.; Lim, Elaine; Rossin, Elizabeth; Kirby, Andrew; Flannick, Jason; Fromer, Menachem; Shakir, Khalid; Fennell, Tim; Garimella, Kiran; Banks, Eric; Poplin, Ryan; Gabriel, Stacey; DePristo, Mark; Wimbish, Jack R.; Boone, Braden E.; Levy, Shawn E.; Betancur, Catalina; Sunyaev, Shamil; Boerwinkle, Eric; Buxbaum, Joseph D.; Cook Jr, Edwin H.; Devlin, Bernie; Gibbs, Richard A.; Roeder, Kathryn; Schellenberg, Gerard D.; Sutcliffe, James S.; Daly, Mark J. (4 April 2012). "Patterns and rates of exonic de novo mutations in autism spectrum disorders". Nature. 485 (7397): 242–245. doi:10.1038/nature11011. PMC 3613847
 . PMID 22495311.
. PMID 22495311. - ↑ Sanders, Stephan J.; Murtha, Michael T.; Gupta, Abha R.; Murdoch, John D.; Raubeson, Melanie J.; Willsey, A. Jeremy; Ercan-Sencicek, A. Gulhan; DiLullo, Nicholas M.; Parikshak, Neelroop N.; Stein, Jason L.; Walker, Michael F.; Ober, Gordon T.; Teran, Nicole A.; Song, Youeun; El-Fishawy, Paul; Murtha, Ryan C.; Choi, Murim; Overton, John D.; Bjornson, Robert D.; Carriero, Nicholas J.; Meyer, Kyle A.; Bilguvar, Kaya; Mane, Shrikant M.; Šestan, Nenad; Lifton, Richard P.; Günel, Murat; Roeder, Kathryn; Geschwind, Daniel H.; Devlin, Bernie; State, Matthew W. (4 April 2012). "De novo mutations revealed by whole-exome sequencing are strongly associated with autism". Nature. 485 (7397): 237–241. doi:10.1038/nature10945.
- ↑ O’Roak, Brian J.; Vives, Laura; Girirajan, Santhosh; Karakoc, Emre; Krumm, Niklas; Coe, Bradley P.; Levy, Roie; Ko, Arthur; Lee, Choli; Smith, Joshua D.; Turner, Emily H.; Stanaway, Ian B.; Vernot, Benjamin; Malig, Maika; Baker, Carl; Reilly, Beau; Akey, Joshua M.; Borenstein, Elhanan; Rieder, Mark J.; Nickerson, Deborah A.; Bernier, Raphael; Shendure, Jay; Eichler, Evan E. (4 April 2012). "Sporadic autism exomes reveal a highly interconnected protein network of de novo mutations". Nature. 485 (7397): 246–250. doi:10.1038/nature10989.
- ↑ Ronemus, Michael; Iossifov, Ivan; Levy, Dan; Wigler, Michael (16 January 2014). "The role of de novo mutations in the genetics of autism spectrum disorders". Nature Reviews Genetics. 15 (2): 133–141. doi:10.1038/nrg3585.
- ↑ Betancur, Catalina (March 2011). "Etiological heterogeneity in autism spectrum disorders: More than 100 genetic and genomic disorders and still counting". Brain Research. 1380: 42–77. doi:10.1016/j.brainres.2010.11.078.
- ↑ SFARI gene https://gene.sfari.org/autdb/Welcome.do. Missing or empty
|title=(help) - ↑ Stefansson, Hreinn; Meyer-Lindenberg, Andreas; Steinberg, Stacy; Magnusdottir, Brynja; Morgen, Katrin; Arnarsdottir, Sunna; Bjornsdottir, Gyda; Walters, G. Bragi; Jonsdottir, Gudrun A.; Doyle, Orla M.; Tost, Heike; Grimm, Oliver; Kristjansdottir, Solveig; Snorrason, Heimir; Davidsdottir, Solveig R.; Gudmundsson, Larus J.; Jonsson, Gudbjorn F.; Stefansdottir, Berglind; Helgadottir, Isafold; Haraldsson, Magnus; Jonsdottir, Birna; Thygesen, Johan H.; Schwarz, Adam J.; Didriksen, Michael; Stensbøl, Tine B.; Brammer, Michael; Kapur, Shitij; Halldorsson, Jonas G.; Hreidarsson, Stefan; Saemundsen, Evald; Sigurdsson, Engilbert; Stefansson, Kari (18 December 2013). "CNVs conferring risk of autism or schizophrenia affect cognition in controls". Nature. 505 (7483): 361–366. doi:10.1038/nature12818.
- ↑ Shinawi, M.; Liu, P.; Kang, S. H. L.; Shen, J.; Belmont, J. W.; Scott, D. A.; Probst, F. J.; Craigen, W. J.; Graham, B. H.; Pursley, A.; Clark, G.; Lee, J.; Proud, M.; Stocco, A.; Rodriguez, D. L.; Kozel, B. A.; Sparagana, S.; Roeder, E. R.; McGrew, S. G.; Kurczynski, T. W.; Allison, L. J.; Amato, S.; Savage, S.; Patel, A.; Stankiewicz, P.; Beaudet, A. L.; Cheung, S. W.; Lupski, J. R. (12 November 2009). "Recurrent reciprocal 16p11.2 rearrangements associated with global developmental delay, behavioural problems, dysmorphism, epilepsy, and abnormal head size". Journal of Medical Genetics. 47 (5): 332–341. doi:10.1136/jmg.2009.073015.
- 1 2 Brandler, William M.; Sebat, Jonathan (14 January 2015). "From De Novo Mutations to Personalized Therapeutic Interventions in Autism". Annual Review of Medicine. 66 (1): 487–507. doi:10.1146/annurev-med-091113-024550.
- ↑ Miyake K, Hirasawa T, Koide T, Kubota T. Epigenetics in autism and other neurodevelopmental diseases. Adv. Exp. Med. Biol.. 2012;724:91–8. doi:10.1007/978-1-4614-0653-2_7. PMID 22411236.
- ↑ Schanen NC. Epigenetics of autism spectrum disorders. Hum. Mol. Genet.. October 2006;15 Spec No 2:R138–50. doi:10.1093/hmg/ddl213. PMID 16987877.
- 1 2 Gardener H, Spiegelman D, Buka SL. Prenatal risk factors for autism: comprehensive meta-analysis. Br J Psychiatry. 2009;195(1):7–14. doi:10.1192/bjp.bp.108.051672. PMID 19567888.
- ↑ Roullet FI, Lai JK, Foster JA. In utero exposure to valproic acid and autism--a current review of clinical and animal studies. Neurotoxicol Teratol. 2013;36:47–56. doi:10.1016/j.ntt.2013.01.004. PMID 23395807.
- 1 2 Libbey JE, Sweeten TL, McMahon WM, Fujinami RS. Autistic disorder and viral infections. J Neurovirol. 2005;11(1):1–10. doi:10.1080/13550280590900553. PMID 15804954.
- ↑ Mendelsohn NJ, Schaefer GB. Genetic evaluation of autism. Semin Pediatr Neurol. 2008;15(1):27–31. doi:10.1016/j.spen.2008.01.005. PMID 18342258.
- ↑ Meyer U, Yee BK, Feldon J. The neurodevelopmental impact of prenatal infections at different times of pregnancy: the earlier the worse?. Neuroscientist. 2007;13(3):241–56. doi:10.1177/1073858406296401. PMID 17519367.
- ↑ Chomiak T, Turner N, Hu B. What We Have Learned about Autism Spectrum Disorder from Valproic Acid. Patholog Res Int. 2013;2013:712758. doi:10.1155/2013/712758. PMID 24381784.
- 1 2 Dufour-Rainfray D, Vourc'h P, Tourlet S, Guilloteau D, Chalon S, Andres CR. Fetal exposure to teratogens: evidence of genes involved in autism. Neurosci Biobehav Rev. 2011;35(5):1254–65. doi:10.1016/j.neubiorev.2010.12.013. PMID 21195109.
- ↑ Miller MT, Strömland K, Ventura L, Johansson M, Bandim JM, Gillberg C. Autism associated with conditions characterized by developmental errors in early embryogenesis: a mini review. Int. J. Dev. Neurosci.. 2005;23(2-3):201–19. doi:10.1016/j.ijdevneu.2004.06.007. PMID 15749246.
- ↑ Román GC. Autism: transient in utero hypothyroxinemia related to maternal flavonoid ingestion during pregnancy and to other environmental antithyroid agents. J Neurol Sci. 2007;262(1–2):15–26. doi:10.1016/j.jns.2007.06.023. PMID 17651757.
- ↑ Xu, Guifeng. Maternal Diabetes and the Risk of Autism Spectrum Disorders in the Offspring: A Systematic Review and Meta-Analysis. Journal of Autism and Developmental Disorders. 22 September 2013;44(4):766–775. doi:10.1007/s10803-013-1928-2. PMID 24057131.
- ↑ Li YM et al.. Association Between Maternal Obesity and Autism Spectrum Disorder in Offspring: A Meta-analysis. J Autism Dev Disord. 2015. doi:10.1007/s10803-015-2549-8. PMID 26254893.
- ↑ Lyall K, Schimdt RJ, Hertz-Picciotto I. Maternal lifestyle and environmental risk factors for autism spectrum disorders. International Journal of Epidemiology. 11 February 2014;43(2):443–464. doi:10.1093/ije/dyt282. PMID 24518932.
- ↑ Kinney DK, Munir KM, Crowley DJ, Miller AM. Prenatal stress and risk for autism. Neurosci Biobehav Rev. 2008;32(8):1519–32. doi:10.1016/j.neubiorev.2008.06.004. PMID 18598714.
- ↑ Fetal testosterone and autistic traits:
- Auyeung B, Baron-Cohen S. A role for fetal testosterone in human sex differences. In: Zimmerman AW. Autism: Current Theories and Evidence. Humana; 2009. doi:10.1007/978-1-60327-489-0_8. ISBN 978-1-60327-488-3. p. 185–208.
- Manson JE. Prenatal exposure to sex steroid hormones and behavioral/cognitive outcomes. Metabolism. 2008;57(Suppl 2):S16–21. doi:10.1016/j.metabol.2008.07.010. PMID 18803959.
- ↑ Abramowicz JS. Ultrasound and autism: association, link, or coincidence?. J Ultrasound Med. 2012;31(8):1261–9. PMID 22837291.
- ↑ Man KK, Tong HH, Wong LY, Chan EW, Simonoff E, Wong IC. Exposure to selective serotonin reuptake inhibitors during pregnancy and risk of autism spectrum disorder in children: A systematic review and meta-analysis of observational studies. Neuroscience and biobehavioral reviews. 9 December 2014;49C:82-89. doi:10.1016/j.neubiorev.2014.11.020. PMID 25498856.
- ↑ Kolevzon A, Gross R, Reichenberg A. Prenatal and perinatal risk factors for autism. Arch Pediatr Adolesc Med. 2007;161(4):326–33. doi:10.1001/archpedi.161.4.326. PMID 17404128.
- ↑ Weisskopf MG, Kioumourtzoglou MA, Roberts AL. Air Pollution and Autism Spectrum Disorders: Causal or Confounded?. Current Environmental Health Reports. December 2015;2(4):430-9. doi:10.1007/s40572-015-0073-9. PMID 26399256.
- ↑ Flores-Pajot MC, Ofner M, Do MT, Lavigne E, Villeneuve PJ. Childhood autism spectrum disorders and exposure to nitrogen dioxide, and particulate matter air pollution: A review and meta-analysis. Environmental Research. 25 August 2016. doi:10.1016/j.envres.2016.07.030. PMID 27609410.
- ↑ Rutter M. Incidence of autism spectrum disorders: changes over time and their meaning. Acta Paediatr. 2005;94(1):2–15. doi:10.1111/j.1651-2227.2005.tb01779.x. PMID 15858952.
- ↑ Schultz RT. Developmental deficits in social perception in autism: the role of the amygdala and fusiform face area. Int J Dev Neurosci. 2005;23(2–3):125–41. doi:10.1016/j.ijdevneu.2004.12.012. PMID 15749240.
- ↑ Ashwood P, Van de Water J. Is autism an autoimmune disease? Autoimmun Rev. 2004;3(7–8):557–62. doi:10.1016/j.autrev.2004.07.036. PMID 15546805.
- ↑ Ashwood P, Wills S, Van de Water J. The immune response in autism: a new frontier for autism research. J Leukoc Biol. 2006;80(1):1–15. doi:10.1189/jlb.1205707. PMID 16698940.
- ↑ Stigler KA, Sweeten TL, Posey DJ, McDougle CJ. Autism and immune factors: a comprehensive review. Res Autism Spectr Disord. 2009;3(4):840–60. doi:10.1016/j.rasd.2009.01.007.
- ↑ Wills S, Cabanlit M, Bennett J, Ashwood P, Amaral D, Van de Water J. Autoantibodies in autism spectrum disorders (ASD). Ann N Y Acad Sci. 2007;1107:79–91. doi:10.1196/annals.1381.009. PMID 17804535.
- ↑ Schmitz C, Rezaie P. The neuropathology of autism: where do we stand? Neuropathol Appl Neurobiol. 2008;34(1):4–11. doi:10.1111/j.1365-2990.2007.00872.x. PMID 17971078.
- ↑ Wu S. Family history of autoimmune diseases is associated with an increased risk of autism in children: A systematic review and meta-analysis.. Neuroscience and biobehavioral reviews. 15 May 2015;55:322-332. doi:10.1016/j.neubiorev.2015.05.004. PMID 25981892.
- ↑ Fox E, Amaral D, Van de Water J. Maternal and fetal antibrain antibodies in development and disease. Dev Neurobiol. 2012;72(10):1327–34. doi:10.1002/dneu.22052. PMID 22911883.
- ↑ Panksepp J. A neurochemical theory of autism. Trends in Neurosciences. 1979;2:174–177. doi:10.1016/0166-2236(79)90071-7.
- ↑ Christison GW, Ivany K. Elimination diets in autism spectrum disorders: any wheat amidst the chaff?. J Dev Behav Pediatr. 2006;27(2 Suppl 2):S162–71. doi:10.1097/00004703-200604002-00015. PMID 16685183.
- ↑ Reichelt KL, Knivsberg A-M, Lind G, Nødland M. Probable etiology and possible treatment of childhood autism. Brain Dysfunct. 1991;4:308–19.
- ↑ Shattock P, Whiteley P. (2002) "Biochemical aspects in autism spectrum disorders: updating the opioid-excess theory and presenting new opportunities for biomedical intervention" "Autism Research Unit, University of Sunderland, UK.
- 1 2 Johnson TW. Dietary considerations in autism: identifying a reasonable approach. Top Clin Nutr. 2006;21(3):212–25. doi:10.1097/00008486-200607000-00008.
- ↑ McElhanon BO, McCracken C, Karpen S, Sharp WG. Gastrointestinal Symptoms in Autism Spectrum Disorder: A Meta-analysis. Pediatrics. 2014;133(5):872–883. doi:10.1542/peds.2013-3995. PMID 24777214.
- ↑ Krishnaswami S, McPheeters ML, Veenstra-Vanderweele J. A systematic review of secretin for children with autism spectrum disorders. Pediatrics. 2011;127(5):e1322–5. doi:10.1542/peds.2011-0428. PMID 21464196.
- ↑ Eyles DW, Burne TH, McGrath JJ. Vitamin D, effects on brain development, adult brain function and the links between low levels of vitamin D and neuropsychiatric disease. Front Neuroendocrinol. 2013;34(1):47–64. doi:10.1016/j.yfrne.2012.07.001. PMID 22796576.
- ↑ Kočovská E, Fernell E, Billstedt E, Minnis H, Gillberg C. Vitamin D and autism: clinical review. Res Dev Disabil. 2012;33(5):1541–50. doi:10.1016/j.ridd.2012.02.015. PMID 22522213.
- 1 2 Zafeiriou DI, Ververi A, Vargiami E. Childhood autism and associated comorbidities. Brain Dev. 2007;29(5):257–72. doi:10.1016/j.braindev.2006.09.003. PMID 17084999.
- ↑ Mehler MF, Purpura DP. Autism, fever, epigenetics and the locus coeruleus. Brain Res Rev. 2009;59(2):388–92. doi:10.1016/j.brainresrev.2008.11.001. PMID 19059284. Lay summary: TIME, 2009-04-07.
- ↑ Austin D. An epidemiological analysis of the 'autism as mercury poisoning' hypothesis. Int J Risk Saf Med. 2008;20(3):135–42. doi:10.3233/JRS-2008-0436.
- ↑ Nelson KB, Bauman ML. Thimerosal and autism?. Pediatrics. 2003;111(3):674–9. doi:10.1542/peds.111.3.674. PMID 12612255.
- ↑ Davidson PW, Myers GJ, Weiss B. Mercury exposure and child development outcomes. Pediatrics. 2004;113(4 Suppl):1023–9. doi:10.1542/peds.113.4.S1.1023. PMID 15060195.
- ↑ Ng DK, Chan CH, Soo MT, Lee RS. Low-level chronic mercury exposure in children and adolescents: meta-analysis. Pediatr Int. 2007;49(1):80–7. doi:10.1111/j.1442-200X.2007.02303.x. PMID 17250511.
- ↑ Ng F, Berk M, Dean O, Bush AI. Oxidative stress in psychiatric disorders: evidence base and therapeutic implications. Int J Neuropsychopharmacol. 2008;11(6):851–76. doi:10.1017/S1461145707008401. PMID 18205981.
- ↑ Kern JK, Jones AM. Evidence of toxicity, oxidative stress, and neuronal insult in autism. J Toxicol Environ Health B Crit Rev. 2006;9(6):485–99. doi:10.1080/10937400600882079. PMID 17090484.
- ↑ Ghanizadeh A, Akhondzadeh S, Hormozi M, Makarem A, Abotorabi-Zarchi M, Firoozabadi A. Glutathione-related factors and oxidative stress in autism, a review. Curr. Med. Chem.. 2012;19(23):4000–5. doi:10.2174/092986712802002572. PMID 22708999.
- ↑ Villagonzalo KA, Dodd S, Dean O, Gray K, Tonge B, Berk M. Oxidative pathways as a drug target for the treatment of autism. Expert Opin. Ther. Targets. 2010;14(12):1301–10. doi:10.1517/14728222.2010.528394. PMID 20954799.
- ↑ Bettelheim B. The Empty Fortress: Infantile Autism and the Birth of the Self. Free Press; 1967. ISBN 0-02-903140-0.
- ↑ Kanner L. Autistic disturbances of affective contact. Nerv Child. 1943;2:217–50. Reprinted in Acta Paedopsychiatr. 1968;35(4):100–36. PMID 4880460.
- ↑ Kanner L. Problems of nosology and psychodynamics in early childhood autism. Am J Orthopsychiatry. 1949;19(3):416–26. doi:10.1111/j.1939-0025.1949.tb05441.x. PMID 18146742.
- ↑ Gardner M. The brutality of Dr. Bettelheim. Skeptical Inquirer. 2000;24(6):12–4.
- ↑ Fombonne E, Zakarian R, Bennett A, Meng L, McLean-Heywood D. Pervasive developmental disorders in Montreal, Quebec, Canada: prevalence and links with immunizations. Pediatrics. 2006;118(1):e139–50. doi:10.1542/peds.2005-2993. PMID 16818529.

- ↑ Gross L. A broken trust: lessons from the vaccine–autism wars. PLoS Biology. 2009;7(5):e1000114. doi:10.1371/journal.pbio.1000114. PMID 19478850.

- ↑ Taylor LE, Swerdfeger AL, Eslick GD. Vaccines are not associated with autism: an evidence-based meta-analysis of case-control and cohort studies. Vaccine. 2014;32(29):3623–9. doi:10.1016/j.vaccine.2014.04.085. PMID 24814559.

- ↑ Hilton S, Petticrew M, Hunt K. 'Combined vaccines are like a sudden onslaught to the body's immune system': parental concerns about vaccine 'overload' and 'immune-vulnerability'. Vaccine. 2006;24(20):4321–7. doi:10.1016/j.vaccine.2006.03.003. PMID 16581162.

- ↑ Gerber JS, Offit PA. Vaccines and autism: a tale of shifting hypotheses. Clinical Infectious Diseases. 2009;48(4):456–61. doi:10.1086/596476. PMID 19128068. Lay summary: IDSA, 2009-01-30.

- ↑ Paul R. Parents ask: am I risking autism if I vaccinate my children?. Journal of Autism and Developmental Disorders. 2009;39(6):962–3. doi:10.1007/s10803-009-0739-y. PMID 19363650.

- 1 2 3 Retraction—Ileal-lymphoid-nodular hyperplasia, non-specific colitis, and pervasive developmental disorder in children. Lancet. 2010-02-06;375(9713):445. doi:10.1016/S0140-6736(10)60175-4. PMID 20137807. Lay summary: BBC News, 2010-02-02.
- ↑ Wakefield A, Murch S, Anthony A et al.. Ileal-lymphoid-nodular hyperplasia, non-specific colitis, and pervasive developmental disorder in children. Lancet. 1998;351(9103):637–41. doi:10.1016/S0140-6736(97)11096-0. PMID 9500320. (Retracted, see doi:10.1016/S0140-6736(10)60175-7)
- ↑ Murch SH, Anthony A, Casson DH et al. Retraction of an interpretation. Lancet. 2004;363(9411):750. doi:10.1016/S0140-6736(04)15715-2. PMID 15016483.
- ↑ Deer B. The MMR-autism crisis - our story so far; 2008-11-02 [Retrieved 2008-12-06].
- 1 2 Centers for Disease Control and Prevention. Measles, mumps, and rubella (MMR) vaccine; 2008-12-23 [Retrieved 2009-02-14].
- 1 2 Institute of Medicine, National Academy of Sciences. Immunization safety review: vaccines and autism; 2004 [Retrieved 2007-06-13].
- ↑ National Health Service. MMR the facts [Retrieved 2007-06-13].
- ↑ Godlee F, Smith J, Marcovitch H. Wakefield's article linking MMR vaccine and autism was fraudulent. BMJ. 2011;342:c7452. doi:10.1136/bmj.c7452. PMID 21209060.
- ↑ Deer B. How the case against the MMR vaccine was fixed. BMJ. 2011;342:c5347. doi:10.1136/bmj.c5347. PMID 21209059.
- ↑ Study linking vaccine to autism was fraud. 2011-01-05 [Retrieved 2011-01-06]. Associated Press. NPR.
- ↑ Retracted autism study an 'elaborate fraud,' British journal finds. (Atlanta) 2011-01-06 [Retrieved 2011-01-06].
- ↑ "Vaccines, blood and biologics: thimerosal in vaccines". US Food and Drug Administration. 2012. Retrieved October 24, 2013.
- ↑ Eaton L. Measles cases in England and Wales rise sharply in 2008. BMJ. 2009;338:b533. doi:10.1136/bmj.b533. PMID 19208716.
- ↑ Choi YH, Gay N, Fraser G, Ramsay M. The potential for measles transmission in England. BMC Public Health. 2008;8:338. doi:10.1186/1471-2458-8-338. PMID 18822142.
- ↑ American Medical Association. AMA Welcomes New IOM Report Rejecting Link Between Vaccines and Autism; 2004-05-18 [Retrieved 2007-07-23].
- ↑ American Academy of Pediatrics. What Parents Should Know About Thimerosal; 2004-05-18 [Retrieved 2007-07-23].
- ↑ Kurt TL. ACMT position statement: the Iom report on thimerosal and autism [PDF]. J Med Toxicol. 2006;2(4):170–1. doi:10.1007/BF03161188. PMID 18072140.
- ↑ Infectious Diseases and Immunization Committee, Canadian Paediatric Society. Autistic spectrum disorder: No causal relationship with vaccines. Paediatr Child Health. 2007 [Retrieved 2008-10-17];12(5):393–5. Also published (2007) in Can J Infect Dis Med Microbiol 18 (3): 177–9. PMID 18923720.
- ↑ "Thimerosal in vaccines". Center for Biologics Evaluation and Research, U.S. Food and Drug Administration. 2007-09-06. Retrieved 2007-10-01.
- ↑ World Health Organization (2006). "Questions and answers about autism spectrum disorders (ASD)". Retrieved 2014-11-02.
- ↑ National Advisory Committee on Immunization. Thimerosal: updated statement. An Advisory Committee Statement. Can Commun Dis Rep. 2007;33(ACS-6):1–13. PMID 17663033.
- ↑ European Medicines Agency. EMEA Public Statement on Thiomersal in Vaccines for Human Use; 2004-03-24 [Retrieved 2007-07-22].
- ↑ Hacking I. The Social Construction of What? Harvard University Press; 1999. ISBN 0-674-00412-4. p. 114–23.
- ↑ Nadesan MH. Constructing Autism: Unravelling the 'Truth' and Understanding the Social. Routledge; 2005. ISBN 0-415-32181-6. The dialectics of autism: theorizing autism, performing autism, remediating autism, and resisting autism. p. 179–213.