Cephalopod size

Cephalopods vary enormously in size. The smallest are only about 1 centimetre (0.39 in) long and weigh less than 1 gram (0.035 oz) at maturity, while the largest—the giant and colossal squids—can exceed 10 m (33 ft) in length and weigh close to half a tonne (1,100 lb), making them the largest living invertebrates. Similarly large cephalopods are known from the fossil record, including enormous examples of ammonoids, belemnoids, nautiloids, and vampyromorphids. In terms of mass, the largest of all known cephalopods were likely the giant shelled ammonoids and endocerids.
Size, and particularly maximum size, has been one of the most interesting aspects of cephalopod science to the general public. This is evidenced by the regular coverage given to the giant squid—and more recently, the colossal squid—in both the popular press and academic literature (see Ellis, 1998; Roper & Shea, 2013; Paxton, 2016). On account of its status as a charismatic megafauna the giant squid has been proposed as an emblematic animal for marine invertebrate conservation (see Guerra et al., 2011).
Certain cephalopod species are noted for having individual body parts of exceptional size. The giant and colossal squids, for example, have the largest known eyes among living animals.
Hatchlings
Hatchlings of Idiosepius thailandicus, possibly the smallest extant cephalopod species at maturity, have a mantle length of around 1 mm (0.039 in) (Nabhitabhata, 1998:32). The closely related Idiosepius pygmaeus weighs only 0.00033 g (1.2×10−5 oz) upon hatching and increases in weight to 0.175 g (0.0062 oz) as it reaches maturity in 50 days (Wood & O'Dor, 2000:93). Even smaller are the hatchlings of the commercially important Illex illecebrosus, with a mass of 0.00015 g (5.3×10−6 oz) (O'Dor et al., 1986:59; Wood & O'Dor, 2000:93). Hatchlings of the giant Pacific octopus (Enteroctopus dofleini)—one of the two largest octopus species—weigh 0.0253 g (0.00089 oz) on average (Cosgrove & McDaniel, 2009:88).
At the other extreme are nautiluses, which upon hatching typically have a shell diameter of 25 mm (0.98 in) or more (depending on the species), the largest hatchling size among extant invertebrates (Grulke, 2014:105). Hatchlings of Nautilus belauensis, one of the larger species, are estimated to weigh on the order of 5.9 g (0.21 oz)[lower-alpha 2] and mature at around 1.2 kg (2.6 lb) after almost 4000 days, or 11 years (Wood & O'Dor, 2000:93).
Smallest adults
The smallest adult size among living cephalopods is attained by the so-called pygmy squids, Idiosepius, and certain diminutive species of the genus Octopus, both of which weigh less than 1 gram (0.035 oz) at maturity (Boletzky, 2003:19). Idiosepius thailandicus is perhaps the smallest of all, with females averaging 10.4 mm (0.41 in) in mantle length and males 5.9 mm (0.23 in) (Nabhitabhata, 1998:28). Average wet weights are around 0.20 g (0.0071 oz) and 0.02 g (0.00071 oz), respectively (Nabhitabhata, 1998:28).
Other tiny species include members of the various Sepiolidae genera; the myopsid squid genera Australiteuthis and Pickfordiateuthis; the oegopsid squid genera Abralia and Abraliopsis; the pygmy cuttlefish Sepia pulchra; and the ram's horn squid, Spirula spirula.
Male dwarfism
The octopod superfamily Argonautoida is characterised by markedly dwarfed males (Boletzky, 1999:24; Boletzky, 2003:20; Norman et al., 2002:733). The four extant genera of the group are Argonauta, Haliphron, Ocythoe, and Tremoctopus, all of which are exclusively pelagic. The greatest disparity in the size of the sexes is seen in the blanket octopuses of the genus Tremoctopus. Norman et al. (2002) reported a fully mature male Tremoctopus violaceus measuring 2.4 cm (0.94 in) in total length and weighing a mere 0.25 g (0.0088 oz). By comparison, the large females of this species reach total lengths of 2 m (6.6 ft) and probably some 10 kg (22 lb) in weight. This is the most extreme sexual size dimorphism known among non-microscopic animals,[lower-alpha 3] with mature females being at least 10,000 times heavier than males, and likely up to 40,000 times heavier (Norman et al., 2002:733; Fairbairn, 2007:3). The related genera Argonauta and Ocythoe have similarly small males, but the females are not nearly as large as those of Tremoctopus, and the size dimorphism is therefore less pronounced. The females of the fourth argonautoid genus, Haliphron, are the largest of all (and possibly the largest octopuses of any kind), but the males are also much larger, at around 30 cm (12 in) (Norman et al., 2002:733).
Extinct taxa
Numerous species of so-called micromorphic ammonites are known (see Kennedy & Cobban, 1990).
Maximum size
The largest living cephalopods in terms of mantle length, total length and mass are all squid, of which the largest species by at least two of these measures is the colossal squid, Mesonychoteuthis hamiltoni. Reaching an estimated 3 m (9.8 ft) in mantle length and 10 m (33 ft) in total length, and weighing as much as 495 kg (1,091 lb),[lower-alpha 4] this species is also the largest of all extant invertebrates (McClain et al., 2015). The only other squid that approaches these dimensions is the giant squid of the genus Architeuthis,[lower-alpha 1] with females up to 275 kg (606 lb), 2.4 m (7.9 ft) in mantle length, and possibly as much as 15 m (49 ft) in total length, making it likely the longest of all cephalopods (McClain et al., 2015). The two largest octopus species—Enteroctopus dofleini and Haliphron atlanticus—can both exceed 70 kg (150 lb), and the former has a maximum total length of more than 6 m (20 ft). Members of the other cephalopod groups are rather small by comparison, although the largest cuttlefish can exceed 10 kg (22 lb) in weight and 50 cm (1.6 ft) in mantle length. Cephalopods of comparable size to the largest present day squid are known from fossil remains, including enormous examples of ammonoids, belemnoids, nautiloids, and vampyromorphids.

The maximum sizes of certain cephalopod species, most notably the giant squid, have often been misreported and exaggerated. Reports of giant squid specimens reaching or even exceeding 18 m (59 ft) in length are widespread, but no animals approaching this size have been scientifically documented in recent times, despite the hundreds of specimens available for study (c. 700 documented as of 2015, of which c. 460 measured in some way; Paxton, 2016). It is now thought likely that such lengths were achieved by great lengthening of the two long feeding tentacles, analogous to stretching elastic bands, or resulted from inadequate measurement methods such as pacing (O'Shea & Bolstad, 2008; Roper & Shea, 2013:113).
More extreme and outlandish giant squid size claims—belonging firmly in the realm of cryptozoology—have appeared in the works of authors such as Willy Ley, Ivan T. Sanderson, and Bernard Heuvelmans (see Sanderson, 1956; Heuvelmans, 1958; Ley, 1959). The existence of these gargantuan squids is often supported by reference to the giant circular scars sometimes found on sperm whales, which are assumed to have been inflicted by the suckers of struggling giant squid. Sometimes these claims are accompanied by extrapolations of body size based on the isometric scaling of a "typical" giant squid. However, such scars are not necessarily of squid origin and may instead represent fungal growths or bite marks, with sea lampreys (Petromyzon marinus) being one possible source (Wood, 1982:193). Even in the case of genuine giant squid sucker marks it is possible that subsequent skin growth has enlarged them well beyond their original dimensions (Wood, 1982:192).[lower-alpha 6]
The literature on cephalopod size has been further muddied by the frequent misattribution of various squid specimens to the giant squid genus Architeuthis, often based solely on their large size. In the academic literature alone, such misidentifications encompass at least the oegopsid families Chiroteuthidae, Cranchiidae, Ommastrephidae, Onychoteuthidae, and Psychroteuthidae[lower-alpha 7] (see Ellis, 1998; Salcedo-Vargas, 1999; Glaubrecht & Salcedo-Vargas, 2004). This situation is further confused by the occasional usage of the common name 'giant squid' in reference to large squid of other genera (see Mitsukuri & Ikeda, 1895; Meek & Goddard, 1926; Clarke & Robson, 1929; Rees, 1950; Nesis, 1970).
Debate has also surrounded the maximum reported dimensions of some other species, including the giant Pacific octopus (Enteroctopus dofleini), with dubious reports of specimens weighing hundreds of kilograms.[lower-alpha 8] The large size of this species made it the focus of octopus wrestling championships, which reached the height of their popularity on the West Coast of the United States in the 1960s (see High, 1976:17; Norman, 2000:217). In contrast to these wholly soft-bodied cephalopods, size determination of the few surviving shelled species (in terms of shell diameter) is comparatively straightforward and can be accomplished with a high level of precision. Whatever the type of cephalopod, in the absence of whole specimens size can often be estimated from only partial remains. For example, cephalopod beaks can be used for mantle length and body weight estimation (see Clarke, 1962; Wolff, 1981; Wolff, 1984; Gröger et al., 2000), and this method has notably been used to estimate the maximum size of the colossal squid.[lower-alpha 9] The lower rostral length (LRL) of the beak is often used for this purpose.
Cephalopod size can be quantified in various ways. Some of the most common size measurements are covered below. The following four tables list only extant species; extinct taxa are treated separately at the end.
Mantle length

Mantle length (ML) is the standard size measure for coleoid cephalopods (shell diameter being more common for nautiluses) and is almost universally reported in the scientific literature. The mantle is the cephalopod's "body"; it lies posterior to the head and encloses the visceral mass and mantle cavity, the latter being used for locomotion by jet propulsion. Unless otherwise indicated, mantle length is measured dorsally over the midline of the mantle. In Decapodiformes (ten-limbed cephalopods), mantle length is measured from the anterior edge of the mantle (near the head), to the posterior end of the mantle or the apex of the united fins, whichever is longer. In Octopodiformes (eight-limbed cephalopods), the anterior edge of the mantle is not clearly delimited dorsally due to advanced head–mantle fusion, and mantle length is therefore taken from the midpoint between the eyes to the posterior end of the mantle. When ventral mantle length is meant instead of dorsal this is always specified as such and abbreviated VML (Roper & Voss, 1983:58).
As an indication of overall size, mantle length is generally considered more reliable than total length because cephalopod limbs may easily be stretched beyond their natural length and are often damaged or missing in preserved specimens (this is particularly true of the long tentacles of many squid species; Glaubrecht & Salcedo-Vargas, 2004:62). Nevertheless, mantle length is not equally applicable to all species. Certain benthic octopuses such as Callistoctopus ornatus are able to elongate and retract their mantles and therefore mantle length measurements, even when taken from a live specimen, may vary considerably. Another problematic case is that of the gelatinous cirroteuthids, whose weakly muscled mantles are prone to substantial shrinkage during preservation. The interocular distance may be a more reliable standard for this group (Roper & Voss, 1983:55).
The list of largest cephalopods by mantle length is dominated by squids, with more than twenty species exceeding the largest-bodied octopuses and cuttlefish. The largest of all is the colossal squid (Mesonychoteuthis hamiltoni) with an estimated maximum mantle length of 3 m (9.8 ft) (Roper & Jereb, 2010c:173). Even greater mantle lengths have historically been reported for the giant squid (Architeuthis dux), but these have been discredited (see O'Shea & Bolstad, 2008).






| Teuthida (squids) | |||
|---|---|---|---|
| Species | Maximum mantle length | References | Notes |
| Mesonychoteuthis hamiltoni (colossal squid) | ~300 cm (estimate) | Roper & Jereb (2010c:173) | The largest complete specimen, a mature female recovered from the Ross Sea in February 2007, had a mantle length of around 2.5 m ([Anonymous], N.d.), and several other specimens near this size have been recorded.[lower-alpha 5] However, at 42.5 mm LRL, its beak is considerably smaller than the largest recovered from a sperm whale stomach (49 mm LRL; [Anonymous], N.d.).[lower-alpha 9] Maximum mantle lengths as great as 4 m have been reported in the past (see for example O'Shea & Bolstad, 2008). There are published claims of a very large section of gladius that would suggest a colossal squid measuring 5 m or more in mantle length (Wood, 1982:191; Bright, 1989:146).[lower-alpha 10] |
| Galiteuthis phyllura | ? 265–275 cm (estimate) | Nesis (1985); Nesis (1987:274); Ellis (1998:149); Glaubrecht & Salcedo-Vargas (2004:65) | Estimate based on 40 cm long arm and 115 cm tentacle from the Sea of Okhotsk.[lower-alpha 11] Roper & Jereb (2010c:165) write: "this is considered a doubtful record that might refer to total length; probably the maximum mantle length is less than 400 to 500 mm". |
| Architeuthis dux[lower-alpha 1] (giant squid) | 240 cm (female) | Landman et al. (2004:686); Roper & Shea (2013:114) | Dorsal mantle length of female captured off Tasmania, Australia, reported by Landman et al. (2004:686) and cited by Roper & Shea (2013:114). Questionable records of up to 500 cm ML can be found in older literature (Roper & Jereb, 2010a:121). O'Shea & Bolstad (2008) give a maximum mantle length of 225 cm based on the examination of more than 130 specimens, as well as beaks recovered from sperm whales (which do not exceed the size of those found in the largest complete specimens). Paxton (2016) accepts a maximum recorded ML of 279 cm, based on the Lyall Bay specimen reported by Kirk (1880:312), but this record has been called into question as the gladius of this specimen was said to be only 190 cm long (Greshko, 2016).[lower-alpha 12]
Including the head and arms but excluding the tentacles (standard length, SL), the species very rarely exceeds 500 cm according to O'Shea & Bolstad (2008). Paxton (2016) considers 945 cm to be the greatest reliably measured SL, based on a specimen reported by Verrill (1880:192), and considers specimens of 10 m SL or more to be "very probable", but these conclusions have been heavily criticised by giant squid experts (Greshko, 2016).[lower-alpha 12] |
| Onykia robusta (robust clubhook squid) | 200 cm | Norman (2000:174); Bolstad (2008:107); Okutani (2015b) | Kubodera et al. (1998) give a maximum of at least 161.5 cm ML. The largest specimen seen by Bolstad (2008:107) had a mantle length of 197 cm (USNM 816872; specimen of indeterminate sex from 51°46.9′N 177°39.7′E / 51.7817°N 177.6617°E). Verrill (1876:237) reported a specimen with a mantle length of 232 cm (91.5 in) and a total length of 4.3 m (14 ft) (excluding the ends of the tentacles, which had been destroyed). Nesis (1987:192) likewise gave a maximum mantle length of 230 cm, but Roper & Jereb (2010h:364) wrote that "this old record might be in error", with the species commonly growing to 160 cm ML. Previously known as Moroteuthis robusta (see Bolstad, 2008; Bolstad, 2010). |
| Megalocranchia maxima | 185 cm (female) | Kubodera & Horikawa (2005:210) | Size of female caught off Motobu Peninsula, Okinawa, Japan, identified as "Megalocranchia cf. maxima" (see Kubodera & Horikawa, 2005:223 for photograph). This species is listed under the name Megalocranchia fisheri in many older sources. Tsuchiya & Okutani (1993), Roper & Jereb (2010c:171) and Okutani (2015a) give maximum of 180 cm, and Norman (2000:158) gives the same for M. fisheri. This species may also be conspecific with Megalocranchia abyssicola (Glaubrecht & Salcedo-Vargas, 2004:65). |
| Taningia danae (Dana octopus squid) | 170 cm | Nesis (1982); Roper & Jereb (2010g:266) | The largest well documented specimen is a 160 cm ML mature female from the North Atlantic (Roper & Vecchione, 1993:449).[lower-alpha 13] |
| Dosidicus gigas (Humboldt squid) | 150 cm | Wormuth (1976:38); Norman (2000:165); Glaubrecht & Salcedo-Vargas (2004:54) | According to Wormuth (1976:38), specimens reaching 150 cm ML are "not uncommon" off Peru. Roper et al. (2010:301) give a maximum mantle length of 120 cm for specimens off Chile and around 100 cm for northern populations, with a more typical mantle length of up to 50–80 cm. The review article of Nigmatullin et al. (2001)—based on c. 230 published papers on the species, in addition to other catch data—also gives a maximum mantle length of 120 cm. |
| Kondakovia longimana (giant warty squid) | ~150 cm (estimate; female) | Bolstad (2008:171) | Estimated size of damaged female (NMV F109447; specimen with 21 mm LRL from 63°04.72′S 62°56.02′E / 63.07867°S 62.93367°E). O'Shea (2003b) estimated maximum mantle length as probably exceeding 115 cm. Largest complete specimen measured 108 cm ML (Lynnes & Rodhouse, 2002:1087; Roper & Jereb, 2010h:366). |
| Mastigoteuthis cordiformis | 100 cm or more | Roper & Jereb (2010f:253) | Based on unpublished reports; largest verified ML is 70 cm (Roper & Jereb, 2010f:253). |
| Lepidoteuthis grimaldii (Grimaldi scaled squid) | 100 cm | Roper & Jereb (2010d:240) | |
| Thysanoteuthis rhombus (diamondback squid) | 100 cm | Nesis (1987:237); Norman (2000:175); Roper et al. (1984); Roper & Jereb (2010j:385) | Commonly grows to 60 cm ML (Roper et al., 1984) and possibly reaches 130 cm ML (Roper & Jereb, 2010j:385). Both sexes are the same size. |
| cf. Magnapinna (bigfin squid) | ~100 cm (estimate) | Vecchione et al. (2001a:2505); Vecchione et al. (2001b) | Estimate based on specimen observed by ROV Tiburon in May 2001, north of Oahu, Hawaii (21°54′N 158°12′W / 21.9°N 158.2°W), at a depth of 3380 m. Its total length was estimated at 4–5 m. |
| Loligo forbesii (veined squid) | 93.7 cm (male) | Jereb et al. (2010:44) | Maximum size of specimens from the Azores. Females from same location grow to 46.2 cm ML. Individuals from the Mediterranean Sea and eastern North Atlantic are usually 20–30 cm ML. |
| Asperoteuthis acanthoderma | 92 cm | Kubodera & Horikawa (2005:209) | Size of specimen (undetermined sex) caught off Motobu Peninsula, Okinawa, Japan (see Kubodera & Horikawa, 2005:223 for photograph). Roper & Jereb (2010b:140) give maximum mantle length of 80 cm. |
| Ommastrephes bartramii (neon flying squid) | 80–90 cm (female) | Roper et al. (2010:296) | Maximum size of specimens from North Atlantic and Southern Hemisphere (where males reach 40–42 cm). Females from the North Pacific are smaller (50–60 cm ML), but males may be larger (40–45 cm ML) (Roper et al., 2010:296). Nesis (1987:231) and Glaubrecht & Salcedo-Vargas (2004:62) give maximum mantle length of 86 cm. |
| Onykia robsoni (rugose hooked squid) | 88.5 cm (female) | Vecchione et al. (2011) | Size of mature female (11.1 kg total weight) caught in bottom trawl at 685–700 m depth over Chatham Rise (44°21′S 175°32′E / 44.350°S 175.533°E). Roper & Jereb (2010h:363) give maximum mantle length of 75 cm. Previously known as Moroteuthis robsoni (see Bolstad, 2008; Bolstad, 2010). |
| Sthenoteuthis oualaniensis (purpleback flying squid) | 82 cm (female) | Roper et al. (2010:317) | Size of exceptionally large mature female of giant form, captured in the Gulf of Guinea (00°58′08″N 02°06′08″E / 0.96889°N 2.10222°E). Males of this giant form reach 24–32 cm ML and females are more typically 36–65 cm. Medium-sized and dwarf forms of this species are also known. |
| Megalocranchia oceanica | 81 cm | Roper & Jereb (2010c:172) | |
| Pholidoteuthis adami | 78 cm | Roper & Jereb (2010i:373) | |
| Todarodes sagittatus | 75 cm | Roper et al. (2010:323) | Size of unsexed specimen from North Atlantic, likely a female. Maximum reported mantle length for males is 64.0 cm, also from North Atlantic. More commonly this species reaches 25.0–35.0 cm ML. |
| Pholidoteuthis massyae | 72 cm | Roper & Jereb (2010i:371) | |
| Octopoda (octopuses) | |||
| Species | Maximum mantle length | References | Notes |
| Haliphron atlanticus (seven-arm octopus) | 69 cm (female) | O'Shea (2002:1); O'Shea (2004:9); Finn (2014a:227) | Measured defrosted and wet, prior to fixing. Isolated beaks of comparable size to that of the present specimen were recorded by Clarke (1986:247–248). The sexually dimorphic males reach a mantle length of over 10 cm (Finn, 2014a:227). |
| Enteroctopus dofleini (giant Pacific octopus) | at least 60 cm | Norman (2000:214); Norman et al. (2014:124) | |
| Sepiida (cuttlefish) | |||
| Species | Maximum mantle length | References | Notes |
| Sepia apama (Australian giant cuttlefish) | 50 cm | Reid et al. (2005:68) | |
| Sepia latimanus (broadclub cuttlefish) | 50 cm | Reid et al. (2005:92) | |
| Sepia hierredda | <50 cm | Reid et al. (2005:88) | |
| Sepia officinalis (European common cuttlefish) | 49 cm | Reid et al. (2005:99) | |
| Sepia pharaonis (pharaoh cuttlefish) | 42 cm | Reid et al. (2005:107) | |
| Sepia lycidas (kisslip cuttlefish) | 38 cm | Reid et al. (2005:96) | |
| Sepia ramani | 37.5 cm | Reid et al. (2005:114) | |
| Vampyromorphida (vampire squid) – single extant species | |||
| Species | Maximum mantle length | References | Notes |
| Vampyroteuthis infernalis (vampire squid) | 13 cm | Nesis (1982); Norman & Finn (2014:269) | |
| Sepiolida (bobtail squids) | |||
| Species | Maximum mantle length | References | Notes |
| Austrorossia antillensis | 9 cm | Reid & Jereb (2005:192) | |
| Rossia pacifica | 9 cm (female) | Reid & Jereb (2005:185) | Males grow to 4.5 cm in mantle length. |
| Rossia macrosoma | 8.5 cm | Reid & Jereb (2005:184) | More typically the mantle length is 2.0–6.0 cm. |
| Neorossia caroli | 8.3 cm (female) | Reid & Jereb (2005:190) | Males grow to 5.1 cm in mantle length. |
| Spirulida (spirula) – single extant species | |||
| Species | Maximum mantle length | References | Notes |
| Spirula spirula (ram's horn squid) | rarely exceeds 4.5 cm | Reid (2005:211) | |
Total length


%2C_Northern_Hawaiian_waters.jpg)
Total length (TL) is measured along the dorsal midline with the limbs outstretched and in line with the body axis. It is the greatest measurable extent of a specimen: from the posterior end of the mantle or fins (or tail, if present; see Arkhipkin et al., 2015) to the apex of the longest limb (Roper & Voss, 1983:58). It is recommended that arms and tentacles be measured in a relaxed state so as not to exaggerate their length, but historically this practice was not always followed and some of the more extreme published giant squid measurements have been attributed to artificial lengthening of the tentacles (O'Shea & Bolstad, 2008). Although total length is often mentioned in relation to the largest cephalopod species, it is otherwise seldom used in teuthology (Roper & Young, 1972:205).
Total length is not to be confused with arm span, also known as radial span, which may be much larger and is often reported for octopuses (for which the arms usually constitute the vast majority of the length). In squids, total length is inclusive of the feeding tentacles, which in some species may be longer than the mantle, head, and arms combined (chiroteuthids such as Asperoteuthis acanthoderma being a prime example).
The longest scientifically documented specimens belong to the giant squid, with a maximum total length of 14–15 m (46–49 ft) (Roper & Shea, 2013:114). Despite its proportionally shorter tentacles, the colossal squid may rival the giant squid in total length, but the species's size limits are uncertain because only a handful of mature specimens have been recorded.[lower-alpha 5]
| Teuthida (squids) | |||
|---|---|---|---|
| Species | Maximum total length | References | Notes |
| Architeuthis dux[lower-alpha 1] (giant squid) | 14–15 m (female) | Roper & Shea (2013:114) | Based on a 40-year data set of more than 50 specimens, Roper & Shea (2013:114) suggest an average total length at maturity of 11 m and a "rarely encountered maximum length" of 14–15 m. Of the nearly 100 specimens examined by Roper, the largest was "46 feet (14 m) long" (Cerullo & Roper, 2012:22). O'Shea & Bolstad (2008) give a maximum total length of 13 m for females based on the examination of more than 130 specimens, measured post mortem and relaxed, as well as beaks recovered from sperm whales (which do not exceed the size of those found in the largest complete specimens). O'Shea estimated the maximum total length for males at 10 m (O'Shea, 2003a).
Older records of 18 m or more were likely exaggerated by stretching of the long feeding tentacles or resulted from inadequate measurement methods such as pacing (O'Shea & Bolstad, 2008; Roper & Shea, 2013:113). Paxton (2016) performed a statistical analysis using literature records of giant squid specimens and concluded that "squid with a conservative TL of 20 m would seem likely based on current data", but the study has been heavily criticised by experts in the field (Greshko, 2016).[lower-alpha 12] |
| Mesonychoteuthis hamiltoni (colossal squid) | approaching 9–10 m (estimate) | Roper & Jereb (2010c:173) | Two specimens of M. hamiltoni recovered from the stomachs of sperm whales between 1956 and 1957 off the South Shetland Islands and South Orkney Islands—both initially identified as Architeuthis—reportedly measured around 10 and 12 m, respectively (Sweeney & Roper, 2001:56; see Korabelnikov, 1959:103 and Yukhov, 1974:62). Estimated maximum lengths as great as 12–14 m have appeared in the popular literature (see Anderton, 2007). |
| cf. Magnapinna (bigfin squid) | ~7 m (estimate) | Vecchione et al. (2001a:2505); Vecchione et al. (2001b); Glaubrecht & Salcedo-Vargas (2004:67); Roper & Jereb (2010e:247) | Estimate based on specimen observed by commercial ROV operated from the oil-drilling ship Millennium Explorer in January 2000, Mississippi Canyon, Gulf of Mexico (28°37′N 88°0′W / 28.617°N 88.000°W), at a depth of 2195 m (Vecchione et al., 2001b). Bolstad (2003) gives an estimate of at least 8 m TL for the largest observed specimen. |
| Asperoteuthis acanthoderma | 5.5 m (+) | Tsuchiya & Okutani (1993) | Total length of immature specimen measuring 0.45 m ML. Much larger specimens of up to 92 cm ML are known (see Kubodera & Horikawa, 2005:223 for photograph). |
| Onykia robusta (robust clubhook squid) | 4.3 m (14 ft) (+) | Verrill (1876:237) | Total length of specimen missing ends of tentacles, with a mantle length of 2.32 m (91.5 in) according to Verrill (1876:237). Glaubrecht & Salcedo-Vargas (2004:66) give maximum total length of 4–6 m. Previously known as Moroteuthis robusta (see Bolstad, 2008; Bolstad, 2010). |
| Galiteuthis phyllura | ? over 4 m (estimate) | Nesis (1985); Ellis (1998:149); Glaubrecht & Salcedo-Vargas (2004:65) | Estimate based on 0.40 m long arm and 1.15 m tentacle from the Sea of Okhotsk.[lower-alpha 11] Roper & Jereb (2010c:165) cast doubt on the validity of this record. |
| Dosidicus gigas (Humboldt squid) | possibly up to 3.7 m (12 ft) | Clarke (1966:117); Glaubrecht & Salcedo-Vargas (2004:59) | Specimens from the northern hemisphere are much smaller, with those off the Californian coast reaching total lengths of less than 1.7 m (Glaubrecht & Salcedo-Vargas, 2004:59). Roper et al. (2010:301) give maximum total length of close to 2.5 m for specimens off Chile. |
| Megalocranchia maxima | 2.7 m (+) (female) | Young & Mangold (2010) | Total length of large female taken off Hawaii (see Young & Mangold, 2010 for photograph). Larger specimens of up to 1.85 m ML have been recorded, and these clearly exceed 2.7 m TL (see Kubodera & Horikawa, 2005:223 for photograph). |
| Taningia danae (Dana octopus squid) | 2.3 m (female) | Roper & Vecchione (1993:444) | Total length of mature female measuring 160 cm in mantle length, taken from frozen specimen.[lower-alpha 13] |
| Kondakovia longimana (giant warty squid) | 2.25 m (+) | Lynnes & Rodhouse (2002:1087) | Size of largest complete specimen (1.08 m ML), found floating at surface off South Orkney Islands (see also Carrington, 2000). Much larger specimens up to an estimated 1.5 m ML are known (Bolstad, 2008:171). |
| Ommastrephes bartramii (neon flying squid) | 2 m | Glaubrecht & Salcedo-Vargas (2004:62) | |
| Octopoda (octopuses) | |||
| Species | Maximum total length | References | Notes |
| Enteroctopus dofleini (giant Pacific octopus) | >6.1 m | Cosgrove (1987) | Norman et al. (2014:124) give the maximum total length as "more than 3 m". Questionable length records of up to 9.8 m can be found in the literature (see High, 1976:18).[lower-alpha 8] |
| Cirrina gen. et sp. indet. | over 4 m (estimate) | Vecchione et al. (2008) | Estimate based on photographic record; finned octopods are known with certainty to reach at least 1.5 m in total length (Vecchione et al., 2008). |
| Haliphron atlanticus (seven-arm octopus) | 4 m (estimate; female) | O'Shea (2004:9); Finn (2014a:227) | Estimate based on incomplete 2.90 m female, measured defrosted and wet, prior to fixing. Isolated beaks of comparable size to that of the present specimen were recorded by Clarke (1986:247–248). Males are estimated to reach a total length of 21 cm (Finn, 2014a:227). |
| Vampyromorphida (vampire squid) – single extant species | |||
| Species | Maximum total length | References | Notes |
| Vampyroteuthis infernalis (vampire squid) | ~30 cm | Norman & Finn (2014:269) | |
Mass
.jpg)



_dark_coloration.jpg)
Cephalopod mass is reported far less frequently than either mantle or total length, and accurate records do not exist for all of the large cephalopod species. It can also vary widely depending on the state of the specimen at the time of weighing (for example, whether it was measured live or dead, wet or dry, frozen or thawed, pre- or post-fixation, with or without egg mass, and so on).
The heaviest known cephalopod, and the largest living invertebrate, is the colossal squid. The largest recorded specimen of this species, caught in the Ross Sea in 2007, weighed 495 kg (1,091 lb). However, its beak was not the largest known for this species; even bigger colossal squid beaks have been recovered from the stomachs of sperm whales, indicating that this species can grow larger still.
| Teuthida (squids) | |||
|---|---|---|---|
| Species | Maximum mass | References | Notes |
| Mesonychoteuthis hamiltoni (colossal squid) | 495 kg (female) | [Anonymous] (N.d.) | Weight of mature female specimen caught in February 2007, measured after thawing. This specimen was originally estimated to weigh 450 kg (Anderton, 2007). Several other specimens with weights in the hundreds of kilograms have been recorded.[lower-alpha 5] Beaks recovered from sperm whale stomachs indicate the existence of even larger specimens, perhaps weighing as much as 600–700 kg ([Anonymous], N.d.).[lower-alpha 9] |
| Architeuthis dux[lower-alpha 1] (giant squid) | 275 kg (female) | O'Shea (2003a) | Maximum size based on the examination of some 105 specimens by O'Shea (2003a), as well as beaks recovered from sperm whales (which do not exceed the size of those found in the largest complete specimens). Maximum weight for males has been estimated at 150 kg (O'Shea, 2003a), though heavier specimens have occasionally been reported (see Deagle et al., 2005 for 190 kg specimen, Hofilena, 2014 for 163 kg specimen). Roper & Jereb (2010a:121) give a maximum weight of up to 500 kg, and "possibly greater". Discredited weights of as much as a tonne or more can be found in older literature (O'Shea & Bolstad, 2008; see for example Alexander, 1998:1233). |
| Taningia danae (Dana octopus squid) | 161.4 kg (female) | Roper & Jereb (2010g:266) | Weight of 160 cm ML mature female from North Atlantic. Specimen weighed prior to freezing (Roper & Vecchione, 1993:444). According to Roper & Jereb (2010g:266), the previously reported maximum weight of 61.4 kg (based on the same specimen) stems from a typographical error in the original paper of Roper & Vecchione (1993).[lower-alpha 13] This lower value was repeated by a number of subsequent authors, including Santos et al. (2001:355) and Kubodera et al. (2006:1029). |
| Onykia robusta (robust clubhook squid) | 50 kg | Roper et al. (1984); Roper & Jereb (2010h:364); Okutani (2015b) | Previously known as Moroteuthis robusta (see Bolstad, 2008; Bolstad, 2010). |
| Dosidicus gigas (Humboldt squid) | 50 kg | Nigmatullin et al. (2001:10); Roper et al. (2010:301); [Anonymous] (N.d.) | Commonly reaches a maximum weight of around 20–30 kg (Roper et al., 2010:301). In their introduction to the family Ommastrephidae, Roper et al. (2010:269) give a maximum weight of 55–65 kg, but this is contradicted later in the same work by the 50 kg figure in the main species account. |
| Thysanoteuthis rhombus (diamondback squid) | 30 kg | Miyahara et al. (2006); Roper & Jereb (2010j:385) | Probably exceeds the recorded mass of 30 kg according to Roper & Jereb (2010j:385). |
| Kondakovia longimana (giant warty squid) | 29 kg | Lynnes & Rodhouse (2002:1087) | Wet weight of largest complete specimen, found floating at surface off South Orkney Islands (see also Carrington, 2000). |
| Ommastrephes bartramii (neon flying squid) | 20–25 kg (female) | Roper et al. (2010:296) | Maximum size of specimens from North Atlantic and Southern Hemisphere (where males reach 2–2.2 kg). Females from the North Pacific are smaller (6 kg), but males may be larger (2–2.9 kg) (Roper et al., 2010:296). |
| Onykia robsoni (rugose hooked squid) | 11.1 kg (female) | Vecchione et al. (2011) | Weight of mature female (88.5 cm ML) caught in bottom trawl at 685–700 m depth over Chatham Rise (44°21′S 175°32′E / 44.350°S 175.533°E). Previously known as Moroteuthis robsoni (see Bolstad, 2008; Bolstad, 2010). |
| Sthenoteuthis oualaniensis (purpleback flying squid) | 8.9 kg | Zuyev et al. (2002:1027) | Roper et al. (2010:315) reported maximum weight of 8.5 kg. |
| Loligo forbesii (veined squid) | 8.3 kg (male) | Jereb et al. (2010:44) | Maximum weight of specimens from the Azores. Females from the same location weigh only up to 2.2 kg. |
| Sthenoteuthis pteropus (orangeback flying squid) | 7 kg | Zuyev et al. (2002:1027); Roper et al. (2010:319) | |
| Octopoda (octopuses) | |||
| Species | Maximum mass | References | Notes |
| Enteroctopus dofleini (giant Pacific octopus) | ? >180 kg | Norman et al. (2014:124) | Cosgrove (1987) and Cosgrove & McDaniel (2009:69) gave a maximum confirmed weight of 71 kg for a live specimen collected in the mid-1960s (McClain et al., 2015). There exists a dubious record of a 272 kg specimen which is sometimes cited as the largest known (see High, 1976:18; Hochberg & Fields, 1980:436; Lewy, 2002:65), although it was never actually collected and weighed (Newman, 1994:66; McClain et al., 2015).[lower-alpha 8] Norman et al. (2014:124) accept a maximum weight of at least 180 kg, which approximates the 182.3 kg reported for a specimen caught off Santa Barbara, California, in 1945, of which photographic evidence survives (Cosgrove & McDaniel, 2009:67–69).[lower-alpha 8] No specimens approaching this size have been reported since the middle of the 20th century, with recent specimens very rarely exceeding 50 kg (Cosgrove & McDaniel, 2009:71). It is possible that the maximum size of the species has decreased over this period, perhaps due to bioaccumulation of toxicants (Anderson, 2003:3; Cosgrove & McDaniel, 2009:71).[lower-alpha 8] |
| Haliphron atlanticus (seven-arm octopus) | 75 kg (estimate) | O'Shea (2004:9) | Estimate based on incomplete 61.0 kg specimen, measured defrosted and wet, prior to fixing. Isolated beaks of comparable size to that of the present specimen were recorded by Clarke (1986:247–248). |
| Sepiida (cuttlefish) | |||
| Species | Maximum mass | References | Notes |
| Sepia apama (Australian giant cuttlefish) | >10.5 kg | Reid et al. (2005:68) | |
| Sepia latimanus (broadclub cuttlefish) | 10 kg | Reid et al. (2005:92) | |
| Sepia hierredda | >7.5 kg | Reid et al. (2005:88) | |
| Sepia lycidas (kisslip cuttlefish) | 5 kg | Reid et al. (2005:96) | |
| Sepia pharaonis (pharaoh cuttlefish) | 5 kg | Reid et al. (2005:107) | |
| Sepia officinalis (European common cuttlefish) | 4 kg | Reid et al. (2005:99) | |
Shell diameter



Nautiluses are the only extant cephalopods with a true external shell; in other groups the shell has been internalised or lost completely. Internal shells include the cuttlebones of cuttlefish, the gladii of squids and the vampire squid, the winged shells of cirrate octopods, and the spiral shells of Spirula. Additionally, females of the octopus genus Argonauta secrete a specialised paper-thin eggcase in which they reside, and this is popularly regarded as a "shell", although it is not attached to the body of the animal (see Finn, 2013).
Cephalopod shell diameter is of interest to teuthologists and conchologists alike. The Registry of World Record Size Shells, the most comprehensive publication on maximum shell size in molluscs, specifies that specimens "should be measured with vernier type calipers and should reflect the greatest measurable dimension of the shell in any direction including any processes of hard shell material produced by the animal (i.e. spines, wings, keels, siphonal canals, etc.) and not including attachments, barnacles, coralline algae, or any other encrusting organisms" (Pisor, 2008:14). Unlike most other measures of cephalopod size, shell diameter can be determined with a high degree of precision and usually leaves little room for ambiguity. For this reason it is usually recorded to the nearest one-tenth of a millimetre (0.0039 in), as is standard in conchology.
When the Registry of World Record Size Shells changed ownership in 2008 it was launched as an online database in addition to its print publication. Subsequent rule changes meant that all records required photographic verification. Over time, older records for which photographic evidence could not be obtained were removed from the database. As a result, some records from older editions of the registry actually exceed the size of the current official record holders, sometimes by considerable margins. Where this has occurred, the largest recorded size across all editions is shown first and any discrepancies or competing records are noted thereafter. Where a reliable literature record surpasses all specimens ever included in the registry, this is given instead and the registry record(s) noted thereafter. Pisor (2008) was the fifth and final print edition of the registry published prior to the rule change, and Barbier et al. (N.d.) is the current, continuously updated online database.
| Octopoda (octopuses) – all extant Argonauta species listed | |||
|---|---|---|---|
| Species | Maximum shell diameter | References | Notes |
| Argonauta argo (greater argonaut) | 300.0 mm | Pisor (2008:22) | Size of a specimen from Australia (registered in 1991; in collection of SIO). Barbier et al. (N.d.) list a record of 256.59 mm for a specimen from the Philippines (year given as 2000; in private collection of G. Munshower).
Under Argonauta cygnus, Pisor (2008:22) listed a 220.0 mm record for a specimen from western Mexico (acquired in 1995; in collection of SIO). The same specimen was previously listed under Argonauta pacifica by Pisor (2005:12). Both A. cygnus and A. pacifica are considered synonyms of A. argo by Finn (2013) and Finn (2014b). |
| Argonauta nodosus (knobbed argonaut) | 292.0 mm | Pisor (2008:22) | Size of a specimen from South Australia (registered in 1977; in collection of AMNH). Barbier et al. (N.d.) list a record of 171.44 mm for a specimen from Tasmania, Australia (year given as 2000; in private collection of G. Munshower). |
| Argonauta hians (muddy argonaut) | 121.61 mm | Barbier et al. (N.d.) | Size of a specimen from the Gulf of Aqaba, Red Sea (year given as 1995; in private collection of Simon Weigmann). Pisor (2008:22) listed a record of 112.6 mm for a specimen from the Philippines (registered in 1988; in private collection of Victor Dan). |
| Argonauta boetgeri[lower-alpha 14] | 108.03 mm | Barbier et al. (N.d.) | Size of a specimen taken from the China Sea off Zhejiang Province by a local fisherman (year given as 2005; in private collection of Simon Weigmann). Pisor (2008:22) listed a record of 102.2 mm for a specimen from the Philippines (collected in 2005; in collection of Havelet Marine). Pisor (2005:12) listed a record of 67.0 mm for a specimen from Mozambique, which is the locus classicus of this species (registered in 2003; in private collection of Pete Stimpson). |
| Argonauta cornutus[lower-alpha 14] | 98.6 mm | Pisor (2008:22) | Size of a specimen from western Mexico (collected in 1999; in private collection of W. D. Schroeder). Barbier et al. (N.d.) list a record of 65.54 mm for a specimen from western Mexico (year given as 2000; in collection of Havelet Marine). |
| Argonauta nouryi | 95.5 mm | Pisor (2008:22); Barbier et al. (N.d.) | Size of a specimen from California (collected in 1992; in collection of Havelet Marine). |
| Nautilida (nautiluses) – all extant species listed | |||
| Species | Maximum shell diameter | References | Notes |
| Nautilus pompilius pompilius (emperor nautilus) | 254.0 mm | Pisor (2008:121); Barbier et al. (N.d.) | Size of a specimen from Indonesia (registered in 2003; in private collection of Pete Stimpson), listed as N. p. pompilius. Hutsell et al. (1997:48) listed a 253.0 mm specimen, also from Indonesia (collected in 1983; in private collection of Cecelia Abbott). Harasewych & Moretzsohn (2010:632) give a maximum shell diameter of 268 mm for this species, but this is based on an erroneous record.[lower-alpha 15]
Under N. repertus (which is treated here in synonymy with N. p. pompilius; Jereb (2005:53) considered it a "questionable species"), Pisor (2005:93) listed a 230.0 mm record for a specimen from Indonesia (registered in 2000; in private collection of Pete Stimpson), Pisor (2008) did not include the taxon at all, while Barbier et al. (N.d.) list a record of 242.07 mm for a specimen from India (no year given; in private collection of Simon Weigmann). The largest albinistic N. p. pompilius is listed by Barbier et al. (N.d.) as a 175.0 mm specimen trawled at 400 m depth off Zamboanga in the Philippines (year given as 2013; in collection of Havelet Marine). |
| Nautilus stenomphalus (white-patch nautilus) | 239.39 mm | Barbier et al. (N.d.) | Size of a specimen from Timor Island, Indonesia (year given as 2009; in private collection of Simon Weigmann). Under N. pompilius f. stenomphalus, Pisor (2008:121) listed a maximum shell diameter of 221.0 mm for a specimen from the Philippines (no year given; in collection of Havelet Marine). |
| Nautilus belauensis (Palau nautilus) | 239 mm | Grulke (2016:76) | Grulke (2016:76) gives an adult shell size range of 180–239 mm, and a mean adult shell diameter of 200 mm. Jereb (2005:54) gives 226 mm as the maximum size for the species, with no reference to a particular specimen. Pisor (2008:121) and Barbier et al. (N.d.) list a record of 221.0 mm for a specimen from Babeldaob, Palau (year given as 1980; in collection of Havelet Marine). |
| Nautilus pompilius suluensis | 220.0 mm | Barbier et al. (N.d.) | Size of a specimen from the Philippines (year given as 2000; in private collection of Pete Stimpson). Pisor (2008:121) listed a maximum shell diameter of 148.0 mm for a specimen from the Philippines (registered in 2000; in private collection of Pete Stimpson). N. p. suluensis is a dwarf form from the Sulu Sea that has the smallest mean shell diameter of all known extant nautilus populations, at 115.6 mm (Dunstan et al., 2011). |
| Allonautilus scrobiculatus (crusty nautilus) | 215.0 mm | Pisor (2005:93) | Size of a specimen from the Philippines (registered in 2000; in private collection of Pete Stimpson), listed as Nautilus scrobiculatus. Pisor (2008) did not include this species at all. Barbier et al. (N.d.) list a record of 214.0 mm for a specimen from Indonesia (year given as 2013; in private collection of Pete Stimpson). |
| Nautilus macromphalus (bellybutton nautilus) | 180.62 mm | Barbier et al. (N.d.) | Size of a specimen from New Caledonia (year given as 2008; in private collection of Simon Weigmann). Pisor (2008:121) listed a maximum shell diameter of 180.0 mm for a specimen from New Caledonia (collected in 1995; in private collection of Kent Trego). |
| Allonautilus perforatus | around 180 mm | Jereb (2005:55); Grulke (2016:83) | Given as the maximum size for the species, with no reference to a particular specimen. Jereb (2005:55) considered A. perforatus a "[v]ery rare form of questionable validity". |
| Spirulida (spirula) – single extant species | |||
| Species | Maximum shell diameter | References | Notes |
| Spirula spirula (ram's horn squid) | 28.8 mm | [Anonymous] (2003c) | Size of a specimen from Samar Island, the Philippines (collected in 2003). Pisor (2008:139) and Barbier et al. (N.d.) list a record of 27.2 mm for a specimen from Phuket Island, Thailand (collected c. 2000; in collection of Havelet Marine; see [Anonymous], 2006 for online record). |
Extinct taxa





Certain extinct cephalopods rivalled or even exceeded the size of the largest living species. In particular, the subclass Ammonoidea is known to have included a considerable number of species that may be considered 'giant' (defined by Stevens (1988) as those exceeding 1 m (3.3 ft) in shell diameter). Heteromorph ammonites are known to have exceeded 1 m in length also, but since their shells were uncoiled to varying degrees, they were overall much smaller than the largest non-heteromorphs. The greatest lengths of all were reached by the orthocones of endocerids such as Cameroceras and Endoceras, which may have exceeded 8 m (26 ft), although their maximum size is uncertain.[lower-alpha 16] However, the uncoiled length of the largest ammonites far exceeds that of even these giant endocerids. Parapuzosia seppenradensis, the largest known ammonite species, had an estimated maximum unrolled shell length of around 18 m (60 ft). It was also possibly the heaviest of all known cephalopods, past or present, with an estimated live mass of 1,456 kg (3,210 lb) (Landois, 1898:27). By comparison, the largest endocerids may have weighed around 1,000 kg (2,200 lb) (Teichert & Kummel, 1960:6). In terms of mass, these are the largest known invertebrates that have ever lived (Grulke, 2014:124).
| Ammonoidea (ammonoids) | |||
|---|---|---|---|
| Species | Maximum shell diameter (length for heteromorphs) | References | Notes |
| Parapuzosia seppenradensis | 2.55 m (estimate) | Landois (1895:100); Landois (1898:27); Teichert & Kummel (1960:6); Summesberger (1979:128); Kennedy & Kaplan (1995:21); Lewy (2002:66) | Widely recognised as the largest ammonite specimen ever found (Payne et al., 2009:27; Grulke, 2014:124). Discovered in 1895 in a quarry in Seppenrade, Coesfeld, Germany, the original is on display at the Westfälisches Museum für Naturkunde in Münster. Estimate based on lectotype measuring 1.742 m in diameter (Kennedy & Kaplan, 1995:21) with an incomplete living chamber, assuming living chamber took up one-fourth of the outer whorl. Teichert & Kummel (1960:6) suggested an even larger shell diameter of around 3.5 m for this specimen, assuming the body chamber extended for three-fourths to one full whorl. Landois (1898:27) estimated the total live weight at 1456 kg, of which the shell would constitute 705 kg. The fossil itself weighs around 3.5 tonnes (Beer, 2015). A smaller specimen of 1.36 m was found in the same quarry some years earlier (Beer, 2015). In 1971 a portion of an ammonite possibly surpassing the Seppenrade specimen was reportedly found in a brickyard in Bottrop, western Germany (Beer, 2015). |
| Parapuzosia bradyi | >1.8 m (estimate) | Larson et al. (1997:44); Lewy (2002:66) | Largest known North American ammonite. Estimate based on incomplete specimen measuring 1.37 m in diameter (missing at least half a whorl of the body chamber). |
| Peltoceratinae gen. et sp. indet. | 1.78 m (estimate) | Poulton (1989) | Estimate based on small portion of outer whorl measuring 1.2 m along the venter and subtending a chord of 1.13 m. The estimate is based on the ultimate whorl height/diameter ratio of "Titanites" occidentalis (about 35%), and assumes a constant rate of expansion. More crude calculations give a circular diameter of 2–2.4 m (best fit of the specimen's outline to a curve yields 2.16 m estimate). |
| Eopachydiscus sp. | 1.67 m | Grulke (2014:125) | This specimen, from the Albian Duck Creek Formation of Texas, has been exhibited at the Tucson Fossil Show and in a New York auction. |
| Pachydesmoceras cf. pachydiscoide | 1.65 m (estimate) | Kin & Niedźwiedzki (2012:19) | Estimate based on 0.98 m diameter specimen representing an apparently complete phragmocone (previously referred to Lewesiceras peramplum or Parapuzosia). A more complete and therefore larger specimen (1.18 m diameter) consisting of a complete phragmocone and near-complete body chamber is also known (Kin & Niedźwiedzki, 2012:17). |
| Lytoceras taharoaense | 1.5 m | Stevens (1985:153); Grulke (2014:126) | Size based on essentially complete shell with only some damage to the aperture. |
| Mesopuzosia mobergi | <1.5 m | Kin & Niedźwiedzki (2012:19) | |
| Parapuzosia austeni | <1.5 m | Kin & Niedźwiedzki (2012:19) | Puzosia mayoriana is a synonym. |
| Moutoniceras sp. | 1.47 m [heteromorph] | Grulke (2014:126) | Likely the largest heteromorph ammonite ever found. Originating from Morocco it is displayed in part of the original rock matrix with sympatric Gassendiceras heteromorphs. Its unrolled shell length would have exceeded 3 m. |
| Parapuzosia bosei | 1.45 m | Scott & Moore (1928:276); Lewy (2002:66) | From the Austin Chalk of the Rio Grande region, Texas. The largest known specimen was reported by Scott & Moore (1928:273) to be 4 ft 9 in (1.45 m) in diameter and "impossible to extract from its matrix". The authors found "[m]any others only slightly smaller", of which three were collected in 1928 and deposited at Texas Christian University (Scott & Moore, 1928:273–274). |
| Parapuzosia americana | 1.37 m (estimate) | Scott & Moore (1928:276) | From the Austin Chalk of the Rio Grande region, Texas. |
| "Titanites" occidentalis | 1.37 m | Frebold (1957:66); Westermann (1966) | Size based on specimen consisting of an imprint and part of the last whorl preserved as an internal mould. |
| Diplomoceras maximum | >1 m [heteromorph] | Olivero & Zinsmeister (1989) | |
| Tropaeum imperator | almost 1 m | Grulke (2014:126) | Largest ammonite known from Australia. Grulke (2014:126) writes: "No exact size is available but it could be almost 1 m across". |
| Belemnoidea (belemnoids) | |||
| Species | Maximum rostrum measurements | References | Notes |
| Megateuthis sp. | 0.7 m TL (Dvms: 30 mm; Dvma: 50 mm) | Schlegelmilch (1998:1); Weis & Mariotti (2007:166); Iba et al. (2015:23–24) | Megateuthis elliptica is "the longest belemnite species known", with rostra from the Humphriesianum Zone in Rumelange and Luxembourg reaching 60–70 cm (Weis & Mariotti, 2007:166). The whole animal is estimated to have been 3–5 m long (Eyden, 2003a). |
| Belemnitina gen. et sp. indet. | ? TL (Dvms: 30 mm; Dvma: ?) | Iba et al. (2015:23) | Known from a single incomplete rostrum (TCSM-J1-0001) from the Pliensbachian Teradani Formation in Teradani, Toyama Prefecture, Japan. The specimen is missing the apical and alveolar regions and comprises only the middle (stem) region of the rostrum. It measures 45 mm in total length by 30 mm and 25 mm across at the anterior and posterior ends, respectively. Iba et al. (2015:23) wrote: "In the Belemnitina, the diameter of the alveolar region is generally larger than those of the apical and stem regions. Thus maximum rostrum diameter of the Teradani specimen is estimated to reach much more than 30 mm." |
| Acroteuthis sp. | ? TL (Dvms: 39 mm; Dvma: 42 mm) | Iba et al. (2015:23) | One of "the largest belemnites ever observed", with a rostrum comparable to that of the indeterminate belemnitinid from Teradani. |
| Pachyteuthis sp. | ? TL (Dvms: 39 mm; Dvma: 40 mm) | Iba et al. (2015:23) | One of "the largest belemnites ever observed", with a rostrum comparable to that of the indeterminate belemnitinid from Teradani. |
| Belemnitina gen. et sp. indet. | ? TL (Dvms: ?; Dvma: >33 mm) | Iba et al. (2015:23) | From the Hettangian Niranohama Formation of northeastern Japan. One of "the largest belemnites ever observed", with a rostrum comparable to but likely slightly smaller than that of the indeterminate belemnitinid from Teradani. |
| Nautiloidea (nautiloids) | |||
| Species | Maximum shell length | References | Notes |
| Endoceras giganteum | 8.15 m (estimate) | Teichert & Kummel (1960:5) | Estimate based on incomplete 3-metre-long shell deposited at the Museum of Comparative Zoology, Harvard University, assuming body chamber-to-phragmocone ratio of 1:2. Teichert & Kummel (1960:2) wrote that this was likely "the largest fragment of an endoceroid cephalopod on display anywhere in the world". The specimen is missing portions of the shell at both ends and it is uncertain whether the specimen includes part of the body chamber (around 50 cm if so) or is entirely phragmocone. It has an adoral diameter of 28 cm, gradually tapering to an adapical diameter of 12 cm. The estimated length of the shell with a complete adapical portion, but not accounting for the unpreserved adoral portion, is 5.8 m. The body chamber alone was estimated to be 2.65 m long (Teichert & Kummel, 1960:5). Klug et al. (2015:270) estimated the total length of the complete shell at 5.733 m, with a volume of 158.6 litres. From the Katian of New York (Klug et al., 2015:270). |
| Cameroceras sp. | 6 m | Frey (1995:73) | Given as maximum size for genus as a whole. |
| Cameroceras proteiforme | 3.0–4.6 m (10–15 ft) | Clarke (1897:778); Teichert & Kummel (1960:1) | Size based on "entire shells" (Clarke, 1897:778). |
| Rayonnoceras solidiforme | 2.8 m (estimate) | Klug et al. (2015:270) | From the Visean of Arkansas. Shell volume estimated at 62.5 litres. |
| Deiroceras hollardi | 2.6 m (estimate) | Klug et al. (2015:270) | From the early Emsian of "Jebel Mdouar". Shell volume estimated at 68.3 litres. |
| Actinocerida gen. et sp. indet. | 1.911 m (estimate) | Klug et al. (2015:270) | From the Llandovery of Gotland. Shell volume estimated at 8.9 litres. |
| Orthocerida gen. et sp. indet. | 1.783 m (estimate) | Klug et al. (2015:270) | From the Ludlow of Gotland. Shell volume estimated at 4.1 litres. |
| Ormoceras TUG 1308-1 | 1.72 m (estimate) | Klug et al. (2015:270) | From the Sandbian of Estonia. Shell volume estimated at 2.7 litres. |
| Ormoceras giganteum MB.C.11940 | 1.71 m (estimate) | Klug et al. (2015:270) | From the Darriwillian. Shell volume estimated at 2.7 litres. |
| Lambeoceras lambii | 1.405 m (estimate) | Leith (1942:130); Teichert & Kummel (1960:4) | Estimate based on incomplete 1.155 m long shell. |
| Orthoceras regarium | 1.39 m (estimate) | Klug et al. (2015:270) | From the Wenlock of Joachimsthal. Shell volume estimated at 5.1 litres. |
| Temperoceras aequinudum | 1.333 m (estimate) | Klug et al. (2015:270) | From the Lochkovian of "Ouidane Chebbi". Shell volume estimated at 9.2 litres. |
| Zeravshanoceras priscum | 1.299 m (estimate) | Klug et al. (2015:270) | From the Eifelian. Shell volume estimated at 1.6 litres. |
| Ordogeisonoceras amplicameratum | >1.25 m | Frey (1995:40) | Shell diameter up to 10.5 cm. Originally described as Orthoceras amplicameratum. Orthoceras ludlowense is considered a synonym. |
| Cameroceras hennepini | <1.2 m (4 ft) (estimate) | Clarke (1897:779) | Size estimate based on "the most complete of the fragments which represent it". |
| Actinoceras vaughanianum | 1.198 m (estimate) | Klug et al. (2015:270) | From the Serpukhovian of Oklahoma. Shell volume estimated at 8.7 litres. |
| Polygrammoceras? cf. P. sp. A | 1.13 m (estimate) | Frey (1995:69) | Estimate based on a "single, very large fragment of a phragmocone". Shell diameter to 9.0 cm. |
| Plagiostomoceras sp. | 1.1 m (estimate) | Klug et al. (2015:270) | From the Givetian of Onondaga, New York. Shell volume estimated at 0.0052 litres. |
| Endoceras decorahense | 1.06 m (estimate; phragmocone only) | Miller & Kummel (1944); Teichert & Kummel (1960:2) | Size estimate based on two portions of an internal mould of the phragmocone, measuring 62.5 cm and 32 cm, with an estimated missing middle section of 11.5 cm. |
| Proterovaginoceras incognitum | 1 m (estimate) | Klug et al. (2015:270) | From the Dapingian of Jämtland, Sweden. Shell volume estimated at 0.8 litres. |
| Teuthida (squids) | |||
| Species | Maximum mantle length | References | Notes |
| Yezoteuthis giganteus | ~1.7 m (estimate) | Tanabe et al. (2006:142) | Size estimate based on preserved upper jaw measuring 97.0 mm in maximum length, similar to that of the largest giant squid (Architeuthis dux). Tanabe et al. (2006:143) wrote that this species "appears to be the largest fossil coleoid ever described". |
| Boreopeltis soniae | 1.3 m+ (estimate) | Eyden (2003b) | Size based on 1.3 m gladius from Queensland, Australia. A second gladius measuring more than a metre and showing possible evidence of predation by Kronosaurus is also known (Eyden, 2003b). |
| Vampyromorphida (vampyromorphids) | |||
| Species | Maximum mantle length | References | Notes |
| Tusoteuthis longa | over 1.8 m (estimate) | Eyden (2003b) | May have reached 5–6 m in total length. Enchoteuthis, Kansasteuthis, and Niobrarateuthis are likely synonyms (Eyden, 2003b). |
Anatomical superlatives
Eyes

The giant and colossal squids have the largest recorded eyes of any living animal, with a maximum diameter of at least 27 cm (11 in) and a 9 cm (3.5 in) pupil (Nilsson et al., 2012:683). This is three times the size of the largest fish eyes—up to 90 mm (3.5 in) in swordfish—and more than twice the diameter of the largest whale eyes—up to 109 mm (4.3 in), 61 mm (2.4 in), and 55 mm (2.2 in) in blue, humpback, and sperm whales, respectively—which are the largest among vertebrates (Nilsson et al., 2012:683). A large colossal squid caught in 2014 and dissected at the Museum of New Zealand Te Papa Tongarewa reportedly had eyes 35 cm (14 in) across (Farquhar, 2014).
Only the extinct ichthyosaurs are known to have approached these dimensions, with some species having eyes up to 35 cm (14 in) in diameter (Nilsson et al., 2012:687). Despite their size, the eyes of giant and colossal squids do not appear to be disproportionately large; they do not deviate significantly from the allometric relationship seen across other squid species (Schmitz et al., 2013:45). Many sources state that the vampire squid (Vampyroteuthis infernalis) has the largest eyes of any animal relative to its size, with a 15 cm (5.9 in) specimen having eyes around 2.5 cm (0.98 in) in diameter (Ellis, 1996:177; though see Young et al., 2015).

Neurons
Squid giant axons can exceed 1 mm (0.039 in) in diameter: 100 to 1000 times the thickness of mammalian axons. The axons of the Humboldt squid (Dosidicus gigas) are exceptional in that they can reach a diameter of as much as 1.5 mm (0.059 in), and those of Loligo forbesii can also exceed 1 mm (Adelman & Gilbert, 1990:102). Such was the importance of Humboldt squid to electrophysiology research that when the animals migrated out of reach of Chilean fishermen in the 1970s "it led to the demise of a world-class electrophysiology laboratory" based there (Scully, 2008). Squid giant axon diameters do not necessarily correlate with overall body size; those of the giant squid (Architeuthis dux) are only 0.137–0.21 mm (0.0054–0.0083 in) thick (Adelman & Gilbert, 1990:102).
The squid giant synapse is the largest chemical junction in nature. It lies in the stellate ganglion on each side of the midline, at the posterior wall of the squid’s muscular mantle. Activation of this synapse triggers a synchronous contraction of the mantle musculature, causing the forceful ejection of a jet of water from the mantle. This water propulsion allows the squid to move rapidly through the water and even to jump through the surface of the water (breaking the air–water barrier) to escape predators. Many essential elements of how all chemical synapses function were first discovered by studying the squid giant synapse (see Llinás, 1999).
Photophores
Taningia danae, a very large octopoteuthid squid, possesses "lemon-sized" yellow photophores at the tips of two of its arms, which are the largest known light-emitting organs in the animal kingdom (Ellis, 1998:149; Barrat, 2015). Video footage shot in 2005 in deep water off Japan shows T. danae emitting blinding flashes of light from these photophores as it attacks its prey (see Kubodera et al., 2006). A pair of muscular lids surrounds each photophore and it is the withdrawal of these lids that produces the flashes. A large individual filmed from a remote submersible off Hawaii in 2015 can clearly be seen opening the lids to reveal its photophores (see Barrat, 2015). It is believed that this highly manoeuvrable squid uses bright flashes to disorientate potential prey. The flashes may also serve to illuminate prey for easier capture or play a role in courtship and/or territorial displays (Kubodera et al., 2006:1033).
Reproductive organs

Extreme penis elongation has been observed in the deep water squid Onykia ingens. When erect, the penis may be as long as the mantle, head, and arms combined (Arkhipkin & Laptikhovsky, 2010:299; Walker, 2010). As such, deep water squids have the greatest known penis length relative to body size of all mobile animals, second in the entire animal kingdom only to certain sessile barnacles (Arkhipkin & Laptikhovsky, 2010:300).
See also
Notes
- 1 2 3 4 5 The taxonomy of the giant squid genus Architeuthis has not been entirely resolved. Lumpers and splitters may propose as many as eight species or as few as one, with most authors recognising either one cosmopolitan species (A. dux) or three geographically disparate species: A. dux from the Atlantic, A. martensi from the North Pacific, and A. sanctipauli from the Southern Ocean (Ellis, 1998:73; Norman, 2000:150; Roper & Jereb, 2010a:121). No genetic or physical basis for distinguishing between the named species has been proposed (Glaubrecht & Salcedo-Vargas, 2004:62), though specimens from the North Pacific do not appear to reach the maximum dimensions seen in giant squid from other areas (Roper & Jereb, 2010a:123). There may also be regional differences in the relative proportions of the tentacles and their sucker counts (see Roeleveld, 2002). The phylogenetic analysis of Winkelmann et al. (2013) supports the existence of a single, globally distributed species (A. dux). The same conclusion was reached by Förch (1998) on the basis of morphological data.
 A large female giant squid (Architeuthis dux) measuring more than 4 m (13 ft) in standard length (length of the mantle, head, and arms, but excluding the long feeding tentacles)
A large female giant squid (Architeuthis dux) measuring more than 4 m (13 ft) in standard length (length of the mantle, head, and arms, but excluding the long feeding tentacles) - ↑ Wood & O'Dor (2000:93) elaborated on this mass estimate as follows:
There are [...] no published weights of hatchling Nautilus spp. The weight of a hatchling N. belauensis was estimated using hatchling shell size and a regression analysis of the cubed shell diameter versus the weight of seven young N. belauensis that weighed <50 g [1.8 oz] [...] in addition to a single hatchling N. pompilius that was weighed for the present study on 24 April 1996 at the Waikiki Aquarium. The hatchling N. pompilius weighing 4.33 g [0.153 oz], with a maximum shell diameter of 26.25 mm [1.033 in], fit a highly significant correlation [...] between cubed shell diameter and weight, which indicates that a hatchling N. belauensis with a 30 mm [1.2 in] shell diameter [...] would weigh approximately 5.9 g [0.21 oz].
- ↑ Norman et al. (2002:733) wrote: "The most extreme examples of sexual size dimorphism come from marine or parasitic taxa where females are difficult to locate (Ghiselin 1974)."
- ↑ By comparison, the live weight of the largest giant clam (Tridacna gigas) specimens is estimated to be in the region of 340 kg (750 lb) (Rosewater, 1965; Knop, 1996; McClain et al., 2015). Several jellyfish species may also rival the mass of the largest squid. One of the top contenders, Nomura's jellyfish (Nemopilema nomurai), grows to around 2 m (6.6 ft) in bell diameter and has a maximum wet weight of some 150–200 kg (330–440 lb) (Omori & Kitamura, 2004; Yasuda, 2004; Kawahara et al., 2006; McClain et al., 2015). Due to their very high water content, however, the dry weight of scyphomedusae is only around 4–9% of their wet weight (Larson, 1986). In squids, dry weight ranges from as much as 26% of wet weight in muscular oceanic species, to less than 9% in some ammoniacal species (see Clarke et al., 1985; Clarke & Goodall, 1994).
- 1 2 3 4 The largest known complete specimen of the colossal squid (Mesonychoteuthis hamiltoni) was a mature female captured in the Ross Sea in February 2007. Its weight was initially estimated at 450 kg (990 lb) and its total length at 8–10 m (26–33 ft) ([Anonymous], N.d.). Once completely thawed the specimen was found to weigh 495 kg (1,091 lb) and measure 2.5 m (8.2 ft) in mantle length, but only 4.2 m (14 ft) in total length ([Anonymous], N.d.). It is likely that the specimen, and particularly its tentacles, shrunk considerably post mortem as a result of dehydration, having been kept in a freezer for 14 months. (As reported by the Museum of New Zealand Te Papa Tongarewa, specimens of Nototodarus sloanii, the New Zealand arrow squid, can shrink by as much as 22% when dehydrated with alcohol solutions; see [Anonymous], N.d..) The colossal squid specimen contracted by a further 5% after several years in preservative fluid (first formalin and later propylene glycol; see Lovis, 2011). A subadult female found in the Ross Sea in March 2003 also had a mantle length of around 2.5 m (8.2 ft), and measured 5.4 m (18 ft) in total length, but was comparatively light at only 300 kg (660 lb) (Griggs, 2003; McClain et al., 2015). Another giant specimen, a female measuring 3.5 m (11 ft) in total length and weighing 350 kg (770 lb), was recovered intact in 2014 (Farquhar, 2014). Other notably large colossal squid specimens include an immature female taken by trawl off Dronning Maud Land in 1981 (2.42 m ML and 5.1 m TL; Ellis, 1998:147), a specimen caught alive in South Georgian waters in 2005 (estimated 5 m TL and 150–200 kg weight; [Anonymous], 2005), and two specimens recovered from sperm whale stomachs between 1956 and 1957 off the South Shetland Islands and South Orkney Islands (reportedly around 10 and 12 m TL, respectively; Sweeney & Roper, 2001:56; see Korabelnikov, 1959:103 and Yukhov, 1974:62).
 The 495-kilogram (1,091 lb) colossal squid specimen on display at the Museum of New Zealand Te Papa Tongarewa
The 495-kilogram (1,091 lb) colossal squid specimen on display at the Museum of New Zealand Te Papa Tongarewa - ↑ A portion of sperm whale skin with giant squid sucker scarsClaims of enormous sucker scars are widespread in the literature. Richard Ellis collected some of "the more egregious examples" in his book, The Search for the Giant Squid (see Ellis, 1998:142). These include the claim of Dozier (1976) that "an ordinary giant squid of 50 feet [15 m] leaves teeth-ringed sucker marks measuring between three and four inches [7.6–10.2 cm] across on a whale, but sperm whales have been captured with tentacle marks 18 inches [46 cm] across." L. Harrison Matthews's monographic treatment of the sperm whale, published in 1938, includes the following: "Nearly all male Sperm whales carry scars caused by the suckers and claws of large squids, scars caused by suckers up to 10 cm. in diameter being common. The claw marks take the form of scratches 2–3 m. in length, and appear to be of more frequent occurrence than sucker marks" (Matthews, 1938). Ellis (1998:142) wrote that this 10 cm figure is "so much larger than any other recorded sucker dimensions that one suspects some sort of error, either in measuring or in transcription." The subject was covered in some detail by Wood (1982:192):
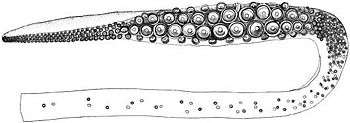 One of the paired tentacular clubs of Architeuthis, showing the enlarged suckers of the manus, which are the largest found on any of the giant squid's limbs
One of the paired tentacular clubs of Architeuthis, showing the enlarged suckers of the manus, which are the largest found on any of the giant squid's limbsMeasurements of 90 ft [27 m], 130 ft [40 m] and even 200 ft [61 m] have been conjectured for giant squids from the size of sucker marks found on the skins of captured sperm whales, but it is dangerous to place too much reliance on this evidence. Verrill says the largest suckers on the tentacles of a 32 ft [9.8 m] long specimen measured 1¼ in [3.2 cm] in diameter, and those on a 52-footer [16 m] about 2 in [5.1 cm]. Daniel (1925), however, examined sucker marks on the head of one cachalot which measured 3½ in [8.9 cm] across, and others measuring up to 5 in [13 cm] in diameter have been found on the skins of sperm whales captured in the North Atlantic. Ivan Sanderson (1956) goes even further and claims that sucker marks over 18 in [46 cm] have been found on the heads of cachalots, but he does not explain how the poor whales managed to escape from the clutches of such colossi! The general consensus of opinion is that exceptionally large sucker marks, i.e. over 2 in [5.1 cm] in diameter, are old scars that have increased in size as the sperm whale grew.
Perhaps the most extreme published claim, ridiculed by Ellis (1998:142), appeared in Willy Ley's 1959 book, Exotic Zoology: "Toothed whales, vomiting in death struggle, have shown evidence of still larger kraken; in one case a 6-foot piece of tentacle, with a diameter of 2 feet [emphasis in original], has been claimed. Another claim goes for marks on the skin of such a whale, looking like the mark of a sucking disk over 2 feet in diameter" (Ley, 1959:210). By comparison, giant squid suckers normally reach a maximum diameter of only a few centimetres. Based on a detailed examination of a number of large specimens from New Zealand waters, Förch (1998:55) wrote that "[t]he largest suckers [...] on the sessile arms are a very constant 21–24 mm in external diameter". In giant squid the largest suckers of all are found on the central portion of the tentacular club, called the manus, and among the specimens examined by Förch (1998:53) these reached a maximum diameter of 28–32 mm. Clarke (1980) wrote: "I have not yet seen conclusive evidence to suggest that sucker scars are larger than 3.7 cm across" (Ellis, 1998:142). - ↑ Iwai (1956:139) reported on two small squid (92 and 104 mm ML) recovered from the "digestive canal" of a sperm whale, which he identified as belonging to the genus Architeuthis. Roper & Young (1972:220) showed that this was certainly a misidentification and attributed them instead to the family Psychroteuthidae. In a brief summary of this case, Ellis (1998:121) gave an erroneous total length of "8 feet" (2.4 m) for the larger of the two specimens. This mistake was repeated by Glaubrecht & Salcedo-Vargas (2004:67), giving rise to the claim of an implausibly large psychroteuthid "with about three meter total length".
- 1 2 3 4 5 6 The maximum size of the giant Pacific octopus (Enteroctopus dofleini) has long been a source of debate in the scientific community. In 1885, reporting on the longest octopus specimen reliably recorded up to that point, renowned malacologist William Healey Dall wrote:
In 1874 I speared an octopus in the harbor of Iliuliuk, Unalashka, which was afterward hung, by a cord tied around the body immediately behind the arms, to one of the stern davits of the coast survey vessel under my command. As soon as the animal died and the muscles relaxed, I noticed that the tips of the longer tentacles just touched the water. On measuring the distance with a cord, I found it to be sixteen feet [4.9 m], giving the creature a spread from tip to tip of the longest pair of arms, of not less than thirty-two feet [9.8 m]. The arms toward the tips were all exceedingly slender, but rather stout toward the body, which was somewhat over a foot [30 cm] long. The largest suckers were two and a half inches [6.4 cm] in diameter ; the whole creature nearly filled a large washtub. Parts of this specimen are now in the U. S. national museum. (Dall, 1885:432)
In an article for the National Marine Fisheries Service summarising knowledge on the giant Pacific octopus, High (1976:17–18) wrote:Several octopuses in excess of 100 pounds [45 kg] have been encountered and captured. Much larger ones have been reported but, like the Loch Ness Monster, these usually elude the careful photographer or scientist. Most octopuses weigh less than 70 pounds [32 kg] with a stretched length of 15 feet [4.6 m] or less. Overall length between arms is not a suitable measure because of the animal's unusual elasticity. In the late 1950's, I interviewed a Canadian commercial diver, Jock MacLean of Prince Rupert, B.C. He reported capturing an immense creature weighing 600 pounds [272 kg] and measuring 32 feet [9.8 m] from arm tip to top. MacLean's photographs, unfortunately, were of poor quality. Smaller animals, to 400 pounds [181 kg], were occasionally taken in his commercial octopus fishing endeavor.
Hochberg & Fields (1980:436) referenced the same specimen, writing: "the largest specimen on record with a total arm spread of 9.6 m [31 ft] and a weight of 272 kg [600 lb]". These figures are only estimates, however, as—contrary to the above quotation from High (1976:17–18)—it appears that this specimen was never collected and measured (McClain et al., 2015). Murray Newman, director of the Vancouver Aquarium for 37 years, quoted Jock MacLean in his 1994 memoir, Life in a Fishbowl: "Next year [1957] in the same place, I saw one, maybe thirty-two feet [9.8 m] across and six hundred pounds [272 kg]. Didn't go for her, though, no place to keep her!" (Newman, 1994:66). Jock MacLean is also reported to have captured a 198 kg (437 lb) animal with a radial span of 8.5 m (28 ft) near Port Hardy, British Columbia, in March 1956 (Newman, 1994:66; Cosgrove & McDaniel, 2009:66–67). Another giant specimen was caught off Santa Barbara, California, in 1945. Its weight was recorded as 182.3 kg (402 lb) and the surviving photograph makes it possible to estimate its total length at more than 3 m (9.8 ft) and radial span at 6–6.7 m (20–22 ft) (Cosgrove & McDaniel, 2009:67–69). In a book dedicated to the giant Pacific octopus, Cosgrove & McDaniel (2009:72) summarised knowledge on the species's maximum size as follows:The specimen William Dall speared in 1885 [sic] at Iliuliuk had the largest radial span of any giant Pacific octopus ever measured. Jock MacLean's 1956 Port Hardy behemoth was the biggest ever weighed. The Santa Barbara specimen photographed in 1945 was the second heaviest. It would appear that octopuses weighing as much as 272 kg (600 lb) and with radial spans of over nine metres (30 ft) are within the realm of possibility, but have never actually been documented by both measuring and weighing.
No specimens approaching these extreme sizes have been reported since the middle of the 20th century. This lack of giant individuals is corroborated by commercial octopus fishers; none of those interviewed have caught a single animal weighing more than 57 kg in the last 20 years, among many thousands harvested over that period (Cosgrove & McDaniel, 2009:71). Octopus specialist Roland Anderson, a biologist with the Seattle Aquarium for more than 30 years, had long sought, unsuccessfully, to find a giant Pacific octopus weighing more than 100 lb (45 kg). In an attempt to raise a truly enormous specimen, Anderson fed a number of captive males ad libitum. The heaviest animal (nicknamed 'Big') attained a peak weight of 43 kg (95 lb) and its largest suckers measured 7.9 cm in diameter (Anderson, 2003:2; Cosgrove & McDaniel, 2009:71). Anderson suggested the species might now be maturing at a smaller size as a result of toxicant bioaccumulation, which could explain the lack of truly gigantic specimens in recent times. In particular, high concentrations of heavy metals and PCBs have been identified in the digestive glands of wild giant Pacific octopuses, likely originating from their preferred prey, the red rock crab (Cancer productus) (Anderson, 2003:3; Cosgrove & McDaniel, 2009:71; Scheel & Anderson, 2012). A preliminary study found that aquarium animals fed equal quantities of raw sea food and live C. productus (caught locally in Elliott Bay) matured at a smaller size, reached a lower maximum weight (27 kg mean), and had higher concentrations of most heavy metals, than those fed solely on raw sea food (36 kg mean, including the aforementioned 43 kg specimen; Anderson, 2003:2). - 1 2 3 Lower rostral length (LRL) is the most widely reported size measure for cephalopod beaks. A submature female colossal squid found in 2003—one of the largest complete specimens ever recovered—weighed around 300 kg (McClain et al., 2015) and had a LRL of 37 mm (O'Shea, 2003c). The largest known specimen of all, captured in 2007, weighed 495 kg and had a LRL of 42.5 mm ([Anonymous], N.d.). The number of large colossal squid specimens known to science is too small to get a good idea of the relationship between beak size and overall body size, but the largest known colossal squid beak from a sperm whale stomach (49 mm LRL) indicates a truly massive animal weighing perhaps as much as 600–700 kg ([Anonymous], N.d.). However, it should be noted that the scaling relationship for this species shows considerable latitude, as demonstrated by a beak of 40 mm LRL extracted from an animal weighing only 160 kg ([Anonymous], N.d.).
 Beak of a colossal squid from the Amundsen Sea off Antarctica
Beak of a colossal squid from the Amundsen Sea off Antarctica - ↑ Wood (1982:191) provided the following details: "Dr Anna M Bidder (pers. comm.) of the Department of Zoology at Cambridge University, possesses a transverse slice of the pen of another Mesonychoteuthis which, judging by its width, must have come from a cranchid measuring at least 5 m [16 ft] in mantle length." The same information is summarised by Bright (1989:146).
- 1 2 Ellis (1998:148–149) wrote of this specimen:
The Russian vessel Novoulianovsk, working in the Sea of Okhotsk in 1984, brought up the remains of a gigantic specimen of Galiteuthis phyllura from a depth of one thousand to thirteen hundred meters (thirty-three hundred to forty-three hundred feet), and Nesis (1985) said that it was "almost as large as Mesonychoteuthis hamiltoni (of the same family)." Only an arm and a tentacle were collected, but they were so large (the arm was 40 cm long [15.6 inches] and the tentacle 115 cm [44.8 inches]) that Nesis was able to estimate the mantle length at 265 to 275 cm (8.61 to 8.93 feet), and the total length at over 4 meters (more than 13 feet). "Because of its narrow body," wrote Nesis, "we conclude that its mass is consistently lower than that of the other large squids."
- 1 2 3
 The "nearly perfect specimen" that was beached alive in Trinity Bay, Newfoundland, on 24 September 1877. A number of exceptionally large giant squid were reported from Newfoundland in the 1870s, and these were meticulously documented in a series of papers by A. E. Verrill.Paxton (2016) compiled data for his statistical analysis from literature records of giant squid specimens. He selected what he regarded as the largest size records for each of mantle length (ML), standard length (SL), and total length (TL). For mantle length, Paxton (2016) considered the 11 ft (3.35 m) reported by Dell (1952:98) as the "longest measured", though "more reliably" the 9 ft 2 in (2.79 m) ML specimen from Lyall Bay, New Zealand, documented by Kirk (1880:312). Paxton added: "A 4.5 m specimen from Mauritius is often mistakenly cited but consultation of the primary paper (Staub, 1993) reveals an ill-defined length which is clearly not ML." The greatest measured ML of a giant squid recovered from a sperm whale is either the 2.4 m reported by Keil (1963:320) (though Paxton writes: "the account is confused and the 2.4 m figure probably refers to the head and ML combined") or the 6 ft 6 in (1.98 m) of a specimen that had been swallowed whole off the Azores, detailed by Clarke (1955:589) and Clarke (1956:257). The "longest visually estimated" ML, according to Paxton, is the c. 100 ft (30 m) of a specimen apparently observed in the North Atlantic, attributed to a personal communication with T. Lipington. A more modest 4 m ML is also given, based on a sighting in the Indian Ocean sourced to the TV documentary of Lynch (2013). For standard length (excluding the tentacles), Paxton (2016) cited the 31 ft (9.45 m) of the "Three Arms specimen" documented by Verrill (1880:192) as the "longest measured". Among specimens recovered from sperm whales, the longest "definitely measured" SL is the 16 ft 3 in (4.95 m) reported by Clarke (1956:257) and the longest "visually estimated" SL is the c. 9 m attributed to a photograph of a sperm whale with giant squid remains in its jaws (see Hansford, 2009), though Paxton conceded that it is "[n]ot clear how much/what portion of body was eaten". For the "longest visually estimated", more extreme supposed SLs of c. 175 ft (53 m) and c. 100 ft (30 m) are cited to Starkey (1963) and Ellis (1998:246), respectively (the latter an eyewitness account by Dennis Braun). Paxton treated these last two size estimates as SLs as opposed to TLs because "squid do not generally leave their tentacles exposed except when grabbing prey and this appears to be the case for Architeuthis". For total length, Paxton (2016) considered three records as candidates for the "longest measured": the 19 m specimen of Berzin (1972:199), the 55 ft 2 in (16.81 m) specimen described by Kirk (1888) as Architeuthis longimanus, and the 55 ft (16.76 m) "Thimble Tickle specimen" reported by Verrill (1880:191). Of the last one, Paxton wrote: "Sometimes mistakenly cited as 17.37 m (57 ft) but the source is clear that it is 55 ft long." The first two records, particularly that of Berzin, are more questionable, as Paxton explained:
The "nearly perfect specimen" that was beached alive in Trinity Bay, Newfoundland, on 24 September 1877. A number of exceptionally large giant squid were reported from Newfoundland in the 1870s, and these were meticulously documented in a series of papers by A. E. Verrill.Paxton (2016) compiled data for his statistical analysis from literature records of giant squid specimens. He selected what he regarded as the largest size records for each of mantle length (ML), standard length (SL), and total length (TL). For mantle length, Paxton (2016) considered the 11 ft (3.35 m) reported by Dell (1952:98) as the "longest measured", though "more reliably" the 9 ft 2 in (2.79 m) ML specimen from Lyall Bay, New Zealand, documented by Kirk (1880:312). Paxton added: "A 4.5 m specimen from Mauritius is often mistakenly cited but consultation of the primary paper (Staub, 1993) reveals an ill-defined length which is clearly not ML." The greatest measured ML of a giant squid recovered from a sperm whale is either the 2.4 m reported by Keil (1963:320) (though Paxton writes: "the account is confused and the 2.4 m figure probably refers to the head and ML combined") or the 6 ft 6 in (1.98 m) of a specimen that had been swallowed whole off the Azores, detailed by Clarke (1955:589) and Clarke (1956:257). The "longest visually estimated" ML, according to Paxton, is the c. 100 ft (30 m) of a specimen apparently observed in the North Atlantic, attributed to a personal communication with T. Lipington. A more modest 4 m ML is also given, based on a sighting in the Indian Ocean sourced to the TV documentary of Lynch (2013). For standard length (excluding the tentacles), Paxton (2016) cited the 31 ft (9.45 m) of the "Three Arms specimen" documented by Verrill (1880:192) as the "longest measured". Among specimens recovered from sperm whales, the longest "definitely measured" SL is the 16 ft 3 in (4.95 m) reported by Clarke (1956:257) and the longest "visually estimated" SL is the c. 9 m attributed to a photograph of a sperm whale with giant squid remains in its jaws (see Hansford, 2009), though Paxton conceded that it is "[n]ot clear how much/what portion of body was eaten". For the "longest visually estimated", more extreme supposed SLs of c. 175 ft (53 m) and c. 100 ft (30 m) are cited to Starkey (1963) and Ellis (1998:246), respectively (the latter an eyewitness account by Dennis Braun). Paxton treated these last two size estimates as SLs as opposed to TLs because "squid do not generally leave their tentacles exposed except when grabbing prey and this appears to be the case for Architeuthis". For total length, Paxton (2016) considered three records as candidates for the "longest measured": the 19 m specimen of Berzin (1972:199), the 55 ft 2 in (16.81 m) specimen described by Kirk (1888) as Architeuthis longimanus, and the 55 ft (16.76 m) "Thimble Tickle specimen" reported by Verrill (1880:191). Of the last one, Paxton wrote: "Sometimes mistakenly cited as 17.37 m (57 ft) but the source is clear that it is 55 ft long." The first two records, particularly that of Berzin, are more questionable, as Paxton explained: The 19-foot (5.8 m) tentacle that fisherman Theophilus Picot hacked off a living animal on 26 October 1873. Picot estimated the total length of the squid at 60 ft (18 m) (Murray, 1874:121).
The 19-foot (5.8 m) tentacle that fisherman Theophilus Picot hacked off a living animal on 26 October 1873. Picot estimated the total length of the squid at 60 ft (18 m) (Murray, 1874:121).The accuracy of the two longest measured TLs of 19 and 16.81 m from a specimen found in the gut of a sperm whale from the Indian Ocean and from the specimen from New Zealand in 1887, respectively, should also be questioned but again are certainly not impossible. The New Zealand specimen (named Architeuthis longimanus Kirk, 1888) clearly has the largest ratio of TL to ML ever known in Architeuthis [...] which led [O'Shea & Bolstad (2008)] to suggest that the length was paced out and/or there was extensive post-mortem stretching. However, a re-reading of the original paper suggests that the specimen, although initially paced out, was actually measured, nevertheless the TL is at the edge of the 99.9% prediction interval range [...] and so it was certainly an unusual specimen. Berzin's (1972) Indian Ocean claim is suspect because of the roundness of the figure, the lack of detailed measurements and because in an associated photo, the mantle (whose length was not given) does not look very large compared to the men in the image. Consequently the measurement, if accurate, would represent another animal with very long tentacles.
However, as Paxton pointed out, the genetic analysis of Winkelmann et al. (2013)—which concluded that there is likely a single, globally-distributed species of Architeuthis—did not encompass these two specimens, and it is therefore possible that there exists a second, as yet unsampled, giant squid species with proportionately longer tentacles. Apart from the somewhat questionable 19 m TL specimen reported by Berzin (1972:199), the longest giant squid recovered from a sperm whale is the 34 ft 5 in (10.49 m) TL individual recorded by Clarke (1956:257) (this specimen also has the longest confirmed ML and SL of any giant squid from a sperm whale). Paxton considered the "longest visually estimated" TL to be the 60 ft (18 m) published by Murray (1874:121), from an eyewitness account by fisherman Theophilus Picot, who claimed to have struck the floating animal from his boat, causing it to attack. Picot managed to hack off one of its tentacles, which was subsequently examined by a number of authors (see Murray, 1874; Verrill, 1875a; Verrill, 1875b). - 1 2 3 The largest well documented specimen of Taningia danae is a 160 cm ML mature female reported by Roper & Vecchione (1993) from the North Atlantic. The original paper gave the mass of this specimen as 61.4 kg (135 lb), but according to Roper & Jereb (2010g:266) this figure is wrong and stems from a typographical error, the correct mass being 161.4 kg (356 lb). Roper & Vecchione (1993) were however consistent in their use of the 61.4 kg figure. At one point they wrote:
 The largest documented specimen of T. danae, preserved at the National Museum of Natural History. It originally weighed 161.4 kg (356 lb) and had a mantle length of 160 cm (5.2 ft).
The largest documented specimen of T. danae, preserved at the National Museum of Natural History. It originally weighed 161.4 kg (356 lb) and had a mantle length of 160 cm (5.2 ft).[...] Zeidler (1981) reported on three large specimens of T. danae found floating dead at the surface by fishermen about 120 km offshore from Port Lincoln, South Australia. One specimen was not retained, but the other two were; one with head and arms missing had a dorsal mantle length of 158 cm and weighed 95 kg, and the other in near-perfect condition was 2.1 m total length (ML not given) and 110 kg. These weights seem excessive compared with our specimen of slightly larger size (61.4 kg, 135 lbs) and we suspect that these weights were incorrectly reported as kg instead of lb. The 158 cm specimen is, to our knowledge, the largest T. danae reported until the 160 cm specimen we record here from the western Atlantic. (Roper & Vecchione, 1993:449)
Another similarly large specimen—a female weighing 124 kg—was reported from northern Spanish waters by González et al. (2003:297) (see also initial reports by [Anonymous], 2000 and Wong, 2000). In July 2010, a sperm whale was photographed off the Azorean island of Faial with a large squid—likely T. danae—in its mouth. The specimen's maximum width, from fin tip to fin tip, was estimated at 1.5–2 m; this would approximate its mantle length (Vecchione et al., 2010). - 1 2 The taxonomic validity of these Argonauta species is questionable. Finn (2013) and Finn (2014b) recognised only four species in the genus: A. argo (syn. A. cygnus, A. pacifica), A. hians (syn. A. boetgeri), A. nodosus, and A. nouryi (syn. A. cornutus).
- ↑ This record is based on a shell collected in 2001 from the Timor Sea off Indonesia, which was sold in 2003 as a specimen of N. repertus with a diameter of 268 mm (10.6 in). It was subsequently found to measure only around 243 mm (9.6 in) and the discrepancy was put down to an encoding mistake ([Anonymous], 2003a). Another specimen from the same locality, sold around the same time, was claimed to measure 255 mm (10.0 in) ([Anonymous], 2003b).
- ↑ While the largest well documented endocerid fossil is likely the 3-metre-long shell fragment housed at the Museum of Comparative Zoology, Harvard University, there are published reports of even larger specimens. Teichert (1927) mentioned specimens up to 5 m long from the Middle Ordovician limestone of Estonia (Teichert & Kummel, 1960:2) and Frey (1995:72) gave a maximum shell length of 6 m for the group. On the subject of endocerid size, Flower (1955:329) wrote:
They are not all large, by any means, but specimens twelve feet [3.7 m] in length have been collected, and fragments of greater diameter indicate a much greater maximum length. I am not wholly inclined to discredit a report of an endoceroid found in a quarry near Watertown New York, which was measured before it was broken up and found to attain a length of 30 feet [9.1 m].
References
- [Anonymous] (2000). Giant Deep-Sea Creature Amazes Spanish Scientists. Reuters, 3 November 2000. [Archived from the original on 1 December 2000.]
- [Anonymous] (2003a). Nautilus repertus ID:118764. Shell Encyclopedia, Conchology, Inc.
- [Anonymous] (2003b). Nautilus repertus ID:118763. Shell Encyclopedia, Conchology, Inc.
- [Anonymous] (2003c). Spirula spirula ID:126690. Shell Encyclopedia, Conchology, Inc.
- [Anonymous] (2005). Very Rare Giant Squid Caught Alive. South Georgia Newsletter, June 2005. [Archived from the original on 16 February 2007.]
- [Anonymous] (2006). Spirula spirula ID:306458. Shell Encyclopedia, Conchology, Inc.
- [Anonymous] (N.d.). Humboldt Squid, Giant Squid, Giant Humboldt Squid, Calamar Gigante, Rojo Diablo (Dosidicus gigas). MexFish.com.
- [Anonymous] (N.d.). How big is the colossal squid? Museum of New Zealand Te Papa Tongarewa.
- [Anonymous] (N.d.). The beak of the colossal squid. Museum of New Zealand Te Papa Tongarewa.
- Adelman, W.J. & D.L. Gilbert (1990). Electrophysiology and Biophysics of the Squid Giant Axon. In: D.L. Gilbert, W.J. Adelman & J.M. Arnold (eds.) Squid as Experimental Animals. Plenum Press, New York. pp. 93–132. ISBN 0306435136.
- Alexander, R.M. (1998). All-time giants: the largest animals and their problems. Palaeontology 41(6): 1231–1245.
- Anderson, R.C. (2003). A preliminary report on bioaccumulation in octopuses (Enteroctopus dofleini). In: T.W. Droscher & D.A. Fraser (eds.) Proceedings of the 2003 Georgia Basin/Puget Sound Research Conference. Puget Sound Partnership, Olympia. 5 pp.
- Anderton, H.J. (2007). World's largest squid landed in NZ. The official website of the New Zealand Government.
- Arkhipkin, A.I. & V.V. Laptikhovsky (2010). Observation of penis elongation in Onykia ingens: implications for spermatophore transfer in deep-water squid. Journal Molluscan Studies 76(3): 299–300. doi:10.1093/mollus/eyq019
- Arkhipkin, A., R. Weis, N. Mariotti & Z. Shcherbich (2015). 'Tailed' cephalopods. Journal of Molluscan Studies 81(3): 345–355. doi:10.1093/mollus/eyu094
- Barbier, J.P., P. Quiquandon & O. Santini (N.d.). Registry of World Record Size Shells. Shells Passion. [Continuously updated; database queried on 22 February 2016.]
- Barrat, J. (2015). Rare squid T. danae captured in new video. Smithsonian Science News, 9 December 2015.
- Beer, M. (2015). Koloss aus der Kreidezeit: Der größte Ammonit der Welt wurde vor 120 Jahren in Seppenrade entdeckt. Westfälische Nachrichten, 22 February 2015. (German)
- Berzin, A.A. (1972). The Sperm Whale. Israel Program for Scientific Translation, Jerusalem. 393 pp. ISBN 0706512626.
- Boletzky, S.v. (1999). Dwarf cephalopods: conditions of reproduction at small size. Berichte der Geologischen Bundesanstalt 46: 24.
- Boletzky, S.v. (2003). A lower limit to adult size in coleoid cephalopods: elements of a discussion. Berliner Paläobiologische Abhandlungen 3: 19–28.
- Bolstad, K.S.R. (2003). Deep-Sea Cephalopods: An Introduction and Overview. The Octopus News Magazine Online.
- Bolstad, K.S.R. (2008). Systematics of the Onychoteuthidae Gray, 1847 (Cephalopoda: Oegopsida). Ph.D. thesis, Auckland University of Technology, Auckland.
- Bolstad, K.S.R. (2010). Systematics of the Onychoteuthidae Gray, 1847 (Cephalopoda: Oegopsida). Zootaxa 2696: 1–186. Preview
- Bright, M. (1989). There are Giants in the Sea: Monsters and Mysteries of the Depths Explored. Robson Books, London. 224 pp. ISBN 0-86051-481-1.
- Carrington, D. (2000). Big squid breaks record. BBC News, 3 July 2000.
- Cerullo, M.M. & C.F.E. Roper (2012). Giant Squid: Searching for a Sea Monster. Capstone Press, North Mankato. 48 pp. ISBN 1429680237.
- Clarke, A., M.R. Clarke, L.J. Holmes & T.D. Waters (1985). Calorific values and elemental analysis of eleven species of oceanic squids (Mollusca: Cephalopoda). Journal of the Marine Biological Association of the United Kingdom 65(4): 983–986. doi:10.1017/S0025315400019457
- Clarke, J.M. (1897). The Lower Silurian Cephalopoda of Minnesota. In: E.O. Ulrich, J.M. Clarke, W.H. Scofield & N.H. Winchell The Geology of Minnesota. Vol. III, Part II, of the final report. Paleontology. Harrison & Smith, Minneapolis. pp. 761–812.
- Clarke, M.R. (1962). The identification of cephalopod "beaks" and the relationship between beak size and total body weight. Bulletin of the British Museum (Natural History), Zoology 8(10): 419–480.
- Clarke, M.R. (1966). A review of the systematics and ecology of oceanic squids. Advances in Marine Biology 4: 91–300. doi:10.1016/S0065-2881(08)60314-4
- Clarke, M.R. (1980). Cephalopoda in the diet of sperm whales of the southern hemisphere and their bearing on sperm whale biology. Discovery Reports 37: 1–324.
- Clarke, M.R. (1986). A Handbook for the Identification of Cephalopod Beaks. Oxford University Press, Oxford. ISBN 0-19-857603-X.
- Clarke, M.R. & N. Goodall (1994). Cephalopods in the diets of three odontocete cetacean species stranded at Tierra del Fuego, Globicephala melaena (Traill, 1809), Hyperoodon planifrons Flower, 1882 and Cephalorhynchus commersonii (Lacepede, 1804). Antarctic Science 6(2): 149–154. doi:10.1017/S0954102094000234
- Clarke, R. (1955). A giant squid swallowed by a sperm whale. Norsk Hvalfangst-Tidende (The Norwegian Whaling Gazette) 44(10): 589–593.
- Clarke, R. (1956). Sperm whales of the Azores. Discovery Report 28: 237–298.
- Clarke, W.J. & G.C. Robson (1929). Notes on the stranding of giant squids on the north-east coast of England. Proceedings of the Malacological Society 18(4): 154–158.
- Cosgrove, J.A. (1987). Aspects of the natural history of Octopus dofleini, the giant Pacific octopus. M.Sc. thesis. Department of Biology, University of Victoria (Canada). 101 pp.
- Cosgrove, J.A. & N. McDaniel (2009). Super Suckers: The Giant Pacific Octopus and Other Cephalopods of the Pacific Coast. Harbour Publishing, Madeira Park. ISBN 1550174665.
- Dall, W.H. (1885). The arms of the octopus, or devil fish. Science 6(145): 432. doi:10.1126/science.ns-6.145.432
- Daniel, R.J. (1925). Animal Life in the Sea. Hodder & Stoughton, London. 119 pp.
- Deagle, B.E., S.N. Jarman, D. Pemberton & N.J. Gales (2005). Genetic screening for prey in the gut contents from a giant squid (Architeuthis sp.). Journal of Heredity 96(4): 417–423. doi:10.1093/jhered/esi036
- Dell, R.K. (1952). The Recent Cephalopoda of New Zealand. Dominion Museum Bulletin 16: 1–157.
- Dozier, T.A. (1976). Dangerous Sea Creatures. Time-Life Television, New York. 128 pp. ISBN 0-913948-04-7.
- Dunstan, A.J., P.D. Ward & N.J. Marshall (2011). Nautilus pompilius life history and demographics at the Osprey Reef Seamount, Coral Sea, Australia. PLoS ONE 6(2): e16312. doi:10.1371/journal.pone.0016312
- Ellis, R. (1996). Deep Atlantic: Life, Death, and Exploration in the Abyss. Knopf, New York. 395 pp. ISBN 0679433244.
- Ellis, R. (1998). The Search for the Giant Squid. Lyons Press, London. 322 pp. ISBN 1-55821-689-8.
- Eyden, P. (2003a). Belemnites: A Quick Look. The Octopus News Magazine Online.
- Eyden, P. (2003b). Tusoteuthis and the Cretaceous Giant Squids. The Octopus News Magazine Online.
- Fairbairn, D.J. (2007). Introduction: the enigma of sexual size dimorphism. In: D.J. Fairbairn, W.U. Blanckenhorn & T. Székely (eds.) Sex, Size and Gender Roles: Evolutionary Studies of Sexual Size Dimorphism. Oxford University Press, Oxford. pp. 1–10. ISBN 0199545588. doi:10.1093/acprof:oso/9780199208784.003.0001
- Farquhar, P. (2014). Scientists Found Only The Second Intact Colossal Squid — Here's What It Looks Like. Business Insider Australia, 16 September 2014.
- Finn, J.K. (2013). Taxonomy and biology of the argonauts (Cephalopoda: Argonautidae) with particular reference to Australian material. Molluscan Research 33(3): 143–222. doi:10.1080/13235818.2013.824854
- Finn, J.K. (2014a). Family Alloposidae. In: P. Jereb, C.F.E. Roper, M.D. Norman & J.K. Finn (eds.) Cephalopods of the world. An annotated and illustrated catalogue of cephalopod species known to date. Volume 3. Octopods and Vampire Squids. FAO Species Catalogue for Fishery Purposes. No. 4, Vol. 3. FAO, Rome. pp. 225–228.
- Finn, J.K. (2014b). Family Argonautidae. In: P. Jereb, C.F.E. Roper, M.D. Norman & J.K. Finn (eds.) Cephalopods of the world. An annotated and illustrated catalogue of cephalopod species known to date. Volume 3. Octopods and Vampire Squids. FAO Species Catalogue for Fishery Purposes. No. 4, Vol. 3. FAO, Rome. pp. 228–237.
- Flower, R.H. (1955). Status of endoceroid classification. Journal of Paleontology 29(3): 329–371. JSTOR 1300321
- Förch, E.C. (1998). The marine fauna of New Zealand: Cephalopoda: Oegopsida: Architeuthidae (giant squid). NIWA Biodiversity Memoir 110: 1–113. ISBN 0-478-08447-1.
- Frebold, H. (1957). "Genus" Titanites Buckman. In: The Jurassic Fernie Group in the Canadian Rocky Mountains and foothills. Geological Survey of Canada Memoir 287. Canada Department of Mines and Technical Surveys. pp. 66–67.
- Frey, R.C. (1995). Middle and Upper Ordovician nautiloid cephalopods of the Cincinnati Arch region of Kentucky, Indiana, and Ohio. U.S. Geological Survey, Denver.
- Ghiselin, M.T. (1974). The Economy of Nature and the Evolution of Sex. University of California Press, Berkeley. 346 pp. ISBN 0-520-02474-5.
- Glaubrecht, M. & M.A. Salcedo-Vargas (2004). The Humboldt squid Dosidicus gigas (Orbigny, 1835): history of the Berlin specimen, with a reappraisal of other (bathy-)pelagic gigantic cephalopods (Mollusca, Ommastrephidae, Architeuthidae). Mitteilungen aus dem Museum für Naturkunde in Berlin, Zoologische Reihe 80(1): 53–69. doi:10.1002/mmnz.20040800105
- González, Á.F., Á. Guerra & F. Rocha (2003). New data on the life history and ecology of the deep-sea hooked squid Taningia danae. Sarsia 88(4): 297–301.
- Greshko, M. (2016). Giant Squid Could Be Bigger Than a School Bus. National Geographic, 27 May 2016.
- Griggs, K. (2003). Super squid surfaces in Antarctic. BBC News, 2 April 2003.
- Gröger, J., U. Piatkowski & H. Heinemann (2000). Beak length analysis of the Southern Ocean squid Psychroteuthis glacialis (Cephalopoda: Psychroteuthidae) and its use for size and biomass estimation. Polar Biology 23(1): 70–74. doi:10.1007/s003000050009
- Grulke, W. (2014). Heteromorph: The rarest fossil ammonites: Nature at its most bizarre. At One Communications. 224 pp. ISBN 978-0-9929740-0-8.
- Grulke, W. (2016). Nautilus: Beautiful survivor: 500 million years of evolutionary history. At One Communications. 224 pp. ISBN 978-0-9929740-2-2.
- Guerra, Á., Á.F. González, S. Pascual & E.G. Dawe (2011). The giant squid Architeuthis: an emblematic invertebrate that can represent concern for the conservation of marine biodiversity. Biological Conservation 144(7): 1989–1997. doi:10.1016/j.biocon.2011.04.021
- Hansford, D. (2009). Rare Photos: Giant Squid Eaten by Sperm Whale. National Geographic News, 29 October 2009.
- Harasewych, M.G. & F. Moretzsohn (2010). The Book of Shells: A lifesize guide to identifying and classifying six hundred shells. A & C Black Publishers, London. ISBN 1408125277.
- Heuvelmans, B. (1958). Dans le sillage des monstres marins: le kraken et le poulpe colossal. Plon, Paris. OCLC 16321559 (French)
- Heuvelmans, B. (2003). The Kraken and the Colossal Octopus: In the Wake of Sea-Monsters. Kegan Paul International, London. xv + 332 pp. ISBN 0-7103-0870-1.
- High, W.L. (1976). The giant Pacific octopus. Marine Fisheries Review 38(9): 17–22.
- Hochberg, F.G. & W.G. Fields (1980). Cephalopoda: The Squids and Octopuses. In: R.H. Morris, D.P. Abbott & E.C. Haderlie (eds.) Intertidal Invertebrates of California. Stanford University Press, Stanford. pp. 429–444. ISBN 0804710457.
- Hofilena, J. (2014). 4-meter long giant squid found caught in fishing net off Japan. The Japan Daily Press, 13 January 2014.
- Hutsell, K.C., L.L. Hutsell & D.L. Pisor (1997). Registry of World Record Size Shells. First edition. Snail's Pace Productions, San Diego. ii + 101 pp. ISBN 0965901718.
- Iba, Y., S.-I. Sano & M. Goto (2015). Large belemnites were already common in the Early Jurassic—new evidence from central Japan. Paleontological Research 19(1): 21–25. doi:10.2517/2014PR025
- Iwai, E. (1956). Descriptions on unidentified species of dibranchiate cephalopods. I. An oegopsiden squid belonging to the genus Architeuthis. The Scientific Reports of the Whale Research Institute 11: 139–151.
- Jereb, P. (2005). Family Nautilidae. In: P. Jereb & C.F.E. Roper, eds. Cephalopods of the world. An annotated and Illustrated catalogue of species known to date. Volume 1. Chambered nautiluses and sepioids (Nautilidae, Sepiidae, Sepiolidae, Sepiadariidae, Idiosepiidae and Spirulidae). FAO Species Catalogue for Fishery Purposes No. 4, Vol. 1. FAO, Rome. pp. 51–55.
- Jereb, P., M. Vecchione & C.F.E. Roper (2010). Family Loliginidae. In: P. Jereb & C.F.E. Roper (eds.) Cephalopods of the world. An annotated and illustrated catalogue of species known to date. Volume 2. Myopsid and Oegopsid Squids. FAO Species Catalogue for Fishery Purposes No. 4, Vol. 2. FAO, Rome. pp. 38–117.
- Kawahara, M., S. Uye, K. Ohtsu & H. Iizumi (2006). Unusual population explosion of the giant jellyfish Nemopilema nomurai (Scyphozoa: Rhizostomeae) in East Asian waters. Marine Ecology Progress Series 307: 161–173. doi:10.3354/meps307161
- Keil, A. (1963). Riesentintenfische aus dem Pottwal-Magen. Natur und Museum 93(8): 319–323. (German)
- Kennedy, W.J. & W.A. Cobban (1990). Cenomanian micromorphic ammonites from the Western Interior of the USA. Palaeontology 33(2): 379–422.
- Kennedy, W.J. & U. Kaplan (1995). Parapuzosia (Parapuzosia) seppenradensis (Landois) und die Ammonitenfauna der Dülmener Schichten, unteres Unter-Campan, Westfalen. Geologie und Paläontologie in Westfalen 33: 1–127. ISBN 3-924590-44-3. (German)
- Kin, A. & R. Niedźwiedzki (2012). First record of the puzosiine ammonite genus Pachydesmoceras from the Middle and Upper Turonian of Poland. Cretaceous Research 33(1): 15–20. doi:10.1016/j.cretres.2011.07.003
- Kirk, T.W. (1880). On the occurrence of giant cuttlefish on the New Zealand coast. Transactions and Proceedings of the New Zealand Institute 12(2): 310–313.
- Kirk, T.W. (1888). Brief description of a new species of large decapod (Architeuthis longimanus). Transactions and Proceedings of the New Zealand Institute 20(1): 34–39.
- Klug, C., K. De Baets, B. Kröger, M.A. Bell, D. Korn & J.L. Payne (2015). Normal giants? Temporal and latitudinal shifts of Palaeozoic marine invertebrate gigantism and global change. Lethaia 48(2): 267–288. doi:10.1111/let.12104
- Knop, D. (1996). Giant Clams: A Comprehensive Guide to the Identification and Care of Tridacnid Clams. Dähne Verlag, Ettlingen.
- Korabelnikov, L.V. (1959). The diet of sperm whales in the Antarctic seas. Priroda 3: 103–104. (Russian)
- Kubodera, T., U. Piatkowski, T. Okutani & M.R. Clarke (1998). Taxonomy and zoogeography of the family Onychoteuthidae (Cephalopoda: Oegopsida). Smithsonian Contributions to Zoology 586(2): 277–291.
- Kubodera, T. & H. Horikawa (2005). Cephalopod fauna around Nansei Islands, southern Japan. In: K. Hasegawa, G. Shinohara & M. Takeda (eds.) Deep-sea Fauna and Pollutants in Nansei Islands. National Science Museum Monographs No. 29. National Science Museum, Tokyo. pp. 191–223. NAID 110004677347
- Kubodera, T., Y. Koyama & K. Mori (2006). Observations of wild hunting behaviour and bioluminescence of a large deep-sea, eight-armed squid, Taningia danae. Proceedings of the Royal Society B: Biological Sciences 274(1613): 1029–1034. doi:10.1098/rspb.2006.0236
- Landman, N.H., J.K. Cochran, R. Cerrato, J. Mak, C.F.E. Roper & C.C. Lu (2004). Habitat and age of the giant squid (Architeuthis sanctipauli) inferred from isotopic analyses. Marine Biology 144(4): 685–691. doi:10.1007/s00227-003-1245-y
- Landois, H. (1895). Die Riesenammoniten von Seppenrade, Pachydiscus Zittel Seppenradensis H. Landois. Jahresbericht des Westfälischen Provinzial-Vereins für Wissenschaft und Kunst 23: 99–108. (German)
- Landois, H. (1898). Gewichtsverhältnisse unserer Riesen-Ammoniten. Jahresbericht des Westfälischen Provinzial-Vereins für Wissenschaft und Kunst 26: 27–28. (German)
- Larson, R.J. (1986). Water content, organic content, and carbon and nitrogen composition of medusae from the northeast Pacific. Journal of Experimental Marine Biology and Ecology 99(2): 107–120. doi:10.1016/0022-0981(86)90231-5
- Larson, N.L., S.D. Jorgensen, R.A. Farrar & P.L. Larson (1997). Ammonites and the Other Cephalopods of the Pierre Seaway. Geoscience Press, Tucson. ISBN 0-945005-25-3.
- Leith, E.I. (1942). Notes on the cephalopod Lambeoceras lambii from Manitoba. Journal of Paleontology 16(1): 130–132.
- Lewy, Z. (2002). The function of the ammonite fluted septal margins. Journal of Paleontology 76(1): 63–69. doi:10.1666/0022-3360(2002)076<0063:TFOTAF>2.0.CO;2
- Ley, W. (1959). Exotic Zoology. The Viking Press, New York. xii + 468 pp. ISBN 067030171X.
- Llinás, R.R. (1999). The Squid Giant Synapse: A Model for Chemical Transmission. Oxford University Press, Oxford. ISBN 0195116526.
- Lovis, P. (2011). Colossal squid on display gets check-up. Te Papa Blog, 14 February 2011.
- Lynch, M. (2013). The Search for the Ocean's Super Predator. Australian Broadcasting Corporation.
- Lynnes, A.S. & P.G. Rodhouse (2002). A big mouthful for predators: the largest recorded specimen of Kondakovia longimana (Cephalopoda: Onychoteuthidae). Bulletin of Marine Science 71(2): 1087–1090.
- Manger, W.L., L.K. Meeks & D.A Stephen (1999). Pathologic Gigantism in Middle Carboniferous Cephalopods, Southern Midcontinent, United States. In: F. Olóriz & F.J. Rodríguez-Tovar (eds.) Advancing Research on Living and Fossil Cephalopods. Kluwer Academic/Plenum Publishers, New York. pp. 77–89. doi:10.1007/978-1-4615-4837-9_7
- Matthews, L.H. (1938). The sperm whale, Physeter catodon. Discovery Reports 17: 93–168.
- McClain, C.R., M.A. Balk, M.C. Benfield, T.A. Branch, C. Chen, J. Cosgrove, A.D.M. Dove, L.C. Gaskins, R.R. Helm, F.G. Hochberg, F.B. Lee, A. Marshall, S.E. McMurray, C. Schanche, S.N. Stone & A.D. Thaler (2015). Sizing ocean giants: patterns of intraspecific size variation in marine megafauna. PeerJ 3: e715. doi:10.7717/peerj.715
- Meek, A. & T.R. Goddard (1926). On two specimens of giant squid stranded on the Northumberland coast. Transactions of the Natural History Society of Northumberland, Durham, and Newcastle-upon-Tyne, new series, 6(2): 229–237.
- Miller, H.W. (1957). Niobrarateuthis bonneri, a new genus and species of squid from the Niobrara Formation of Kansas. Journal of Paleontology 31(4): 809–811. JSTOR 1300484
- Miller, A.K. & B. Kummel (1944). Some large straight Ordovician cephalopods from Minnesota. Annals of the Carnegie Museum 30(2): 19–38.
- Mitsukuri, K. & S. Ikeda (1895). Notes on a gigantic cephalopod. Zoological Magazine 7(77): 39–50. (Japanese)
- Miyahara, K., K. Fukui, T. Ota & T. Minami (2006). Laboratory observations on the early life stages of the diamond squid Thysanoteuthis rhombus. Journal of Molluscan Studies 72(2): 199–205. doi:10.1093/mollus/eyi068
- Murray, A. (1874). Capture of a gigantic squid at Newfoundland. American Naturalist 8(2): 120–123. [See also correction: Gigantic cuttle-fishes of Newfoundland. American Naturalist 8(4): 226–227.]
- Nabhitabhata, J. (1998). Distinctive behaviour of Thai pygmy squid, Idiosepius thailandicus Chotiyaputta, Okutani & Chaitiamvong, 1991. Phuket Marine Biological Center Special Publication 18(1): 25–40.
- Nesis, K.N. (1970). Biology of the Peru–Chilean giant squid, Dosidicus gigas. Okeanologiya 10(1): 140–152. (Russian)
- Nesis, K.N. (1982). Abridged key to the cephalopod mollusks of the world's ocean. Light and Food Industry Publishing House, Moscow. 385+ii pp. (Russian) [Translated into English by B.S. Levitov, ed. by L.A. Burgess (1987). (1987). Cephalopods of the World: Squids, Cuttlefishes, Octopuses, and Allies. T.F.H. Publications, Neptune City.]
- Nesis, K.N. (1985). A giant squid in the Sea of Okhotsk. Priroda 10: 112–113. (Russian)
- Nesis, K.N. (1987). Cephalopods of the World: Squids, Cuttlefishes, Octopuses, and Allies. T.F.H. Publications, Neptune City. ISBN 0-86622-051-8.
- Newman, M. (1994). Life in a Fishbowl: Confessions of an Aquarium Director. Douglas & McIntyre, Vancouver. ix + 262 pp. ISBN 1550541250.
- Nicholls, E.L. & H. Isaak (1987). Stratigraphic and taxonomic significance of Tusoteuthis longa Logan (Coleoidea, Teuthida) from the Pembina Member, Pierre Shale (Campanian), of Manitoba. Journal of Paleontology 61(4): 727–737. doi:10.1017/S0022336000029085
- Nigmatullin, C.M., K.N. Nesis & A.I. Arkhipkin (2001). A review of the biology of the jumbo squid Dosidicus gigas (Cephalopoda: Ommastrephidae). Fisheries Research 54(1): 9–19. doi:10.1016/S0165-7836(01)00371-X
- Nilsson, D.-E., E.J. Warrant, S. Johnsen, R. Hanlon & N. Shashar (2012). A unique advantage for giant eyes in giant squid. Current Biology 22(8): 683–688. doi:10.1016/j.cub.2012.02.031
- Norman, M.D. (2000). Cephalopods: A World Guide. ConchBooks, Hackenheim. 320 pp. ISBN 3-925919-32-5.
- Norman, M.D. & J.K. Finn (2014). Family Vampyroteuthidae. In: P. Jereb, C.F.E. Roper, M.D. Norman & J.K. Finn (eds.) Cephalopods of the world. An annotated and illustrated catalogue of cephalopod species known to date. Volume 3. Octopods and Vampire Squids. FAO Species Catalogue for Fishery Purposes. No. 4, Vol. 3. FAO, Rome. pp. 268–270.
- Norman, M.D., J.K. Finn & F.G. Hochberg (2014). Family Octopodidae. In: P. Jereb, C.F.E. Roper, M.D. Norman & J.K. Finn (eds.) Cephalopods of the world. An annotated and illustrated catalogue of cephalopod species known to date. Volume 3. Octopods and Vampire Squids. FAO Species Catalogue for Fishery Purposes. No. 4, Vol. 3. FAO, Rome. pp. 36–215.
- Norman, M.D., D. Paul, J. Finn & T. Tregenza (2002). First encounter with a live male blanket octopus: the world's most sexually size‐dimorphic large animal. New Zealand Journal of Marine and Freshwater Research 36(4): 733–736. doi:10.1080/00288330.2002.9517126
- O'Dor, R.K., E.A. Foy, P.L. Helm & N. Balch (1986). The locomotion and energetics of hatchling squid, Illex illecebrosus. American Malacological Bulletin 4(1): 55–60.
- Okutani, T. (1995). Cuttlefish and squids of the world in color. Publication for the 30th anniversary of the foundation of the National Cooperative Association of Squid Processors. 185 pp.
- Okutani, T. (2015a). Megalocranchia maxima Pfeffer, 1884. In: Cuttlefishes and Squids of the World: New Edition. National Cooperative Association of Squid Processors. 246 pp. ISBN 4486037340.
- Okutani, T. (2015b). Onykia (Onykia) robusta (Verrill, 1876). In: Cuttlefishes and Squids of the World: New Edition. National Cooperative Association of Squid Processors. 246 pp. ISBN 4486037340.
- Olivero, E.B. & W.J. Zinsmeister (1989). Large heteromorph ammonites from the Upper Cretaceous of Seymour Island, Antarctica. Journal of Paleontology 63(5): 626–636. JSTOR 1305622
- Omori, M. & M. Kitamura (2004). Taxonomic review of three Japanese species of edible jellyfish (Scyphozoa: Rhizostomeae). Plankton Biology & Ecology 51(1): 36–51.
- O'Shea, S. (2002). Haliphron atlanticus — a giant gelatinous octopus. Biodiversity Update 5: 1.
- O'Shea, S. (2003a). Giant Squid and Colossal Squid Fact Sheet. The Octopus News Magazine Online. [Archived from the original on 7 December 2003.]
- O'Shea, S. (2003b). Kondakovia longimana. In: Giant Squid and Colossal Squid Fact Sheet. The Octopus News Magazine Online. [Archived from the original on 27 April 2006.]
- O'Shea, S. (2003c). Re: Colossal Squid Necropsy. The Octopus News Magazine Online Forums, 15 August 2003.
- O'Shea, S. (2004). The giant octopus Haliphron atlanticus (Mollusca : Octopoda) in New Zealand waters. New Zealand Journal of Zoology 31(1): 7–13. doi:10.1080/03014223.2004.9518353
- O'Shea, S. & K. Bolstad (2008). Giant Squid and Colossal Squid Fact Sheet. The Octopus News Magazine Online.
- Paxton, C.G.M. (2016). Unleashing the Kraken: on the maximum length in giant squid (Architeuthis sp.). Journal of Zoology 300(2): 82–88. doi:10.1111/jzo.12347
- Payne, J.L., A.G. Boyer, J.H. Brown, S. Finnegan, M. Kowalewski, R.A. Krause, Jr., S.K. Lyons, C.R. McClain, D.W. McShea, P.M. Novack-Gottshall, F.A. Smith, J.A. Stempien & S.C. Wang (2009). Two-phase increase in the maximum size of life over 3.5 billion years reflects biological innovation and environmental opportunity. PNAS 106(1): 24–27. doi:10.1073/pnas.0806314106
- Pisor, D.L. (2005). Registry of World Record Size Shells. Fourth edition. ConchBooks, Hackenheim. 171 pp. ISBN 0965901742.
- Pisor, D.L. (2008). Registry of World Record Size Shells. Fifth edition. ConchBooks, Hackenheim. 207 pp. ISBN 0615194753.
- Poulton, T.P. (1989). Jurassic (Oxfordian) ammonites from the Fernie Formation of Western Canada: a giant peltoceratinid, and Cardioceras canadense Whiteaves. Contributions to Canadian Paleontology, Geological Survey of Canada, Bulletin 396: 173–179.
- Rees, W.J. (1950). On a giant squid, Ommastrephes caroli Furtado stranded at Looe, Cornwall. Bulletin of the British Museum (Natural History), Zoology 1(2): 29–42.
- Reid, A. (2005). Family Spirulidae. In: P. Jereb & C.F.E. Roper, eds. Cephalopods of the world. An annotated and illustrated catalogue of species known to date. Volume 1. Chambered nautiluses and sepioids (Nautilidae, Sepiidae, Sepiolidae, Sepiadariidae, Idiosepiidae and Spirulidae). FAO Species Catalogue for Fishery Purposes No. 4, Vol. 1. FAO, Rome. pp. 211–212.
- Reid, A. & P. Jereb (2005). Family Sepiolidae. In: P. Jereb & C.F.E. Roper, eds. Cephalopods of the world. An annotated and illustrated catalogue of species known to date. Volume 1. Chambered nautiluses and sepioids (Nautilidae, Sepiidae, Sepiolidae, Sepiadariidae, Idiosepiidae and Spirulidae). FAO Species Catalogue for Fishery Purposes No. 4, Vol. 1. FAO, Rome. pp. 153–203.
- Reid, A., P. Jereb, & C.F.E. Roper (2005). Family Sepiidae. In: P. Jereb & C.F.E. Roper, eds. Cephalopods of the world. An annotated and illustrated catalogue of species known to date. Volume 1. Chambered nautiluses and sepioids (Nautilidae, Sepiidae, Sepiolidae, Sepiadariidae, Idiosepiidae and Spirulidae). FAO Species Catalogue for Fishery Purposes No. 4, Vol. 1. FAO, Rome. pp. 57–152.
- Roeleveld, M.A.C. (2000). Giant squid beaks: implications for systematics. Journal of the Marine Biological Association of the United Kingdom 80(1): 185–187. doi:10.1017/S0025315499001769
- Roeleveld, M.A.C. (2002). Tentacle morphology of the giant squid Architeuthis from the North Atlantic and Pacific Oceans. Bulletin of Marine Science 71(2): 725–737.
- Roper, C.F.E. & P. Jereb (2010a). Family Architeuthidae. In: P. Jereb & C.F.E. Roper (eds.) Cephalopods of the world. An annotated and illustrated catalogue of species known to date. Volume 2. Myopsid and Oegopsid Squids. FAO Species Catalogue for Fishery Purposes No. 4, Vol. 2. FAO, Rome. pp. 121–123.
- Roper, C.F.E. & P. Jereb (2010b). Family Chiroteuthidae. In: P. Jereb & C.F.E. Roper (eds.) Cephalopods of the world. An annotated and illustrated catalogue of species known to date. Volume 2. Myopsid and Oegopsid Squids. FAO Species Catalogue for Fishery Purposes No. 4, Vol. 2. FAO, Rome. pp. 135–145.
- Roper, C.F.E. & P. Jereb (2010c). Family Cranchiidae. In: P. Jereb & C.F.E. Roper (eds.) Cephalopods of the world. An annotated and illustrated catalogue of species known to date. Volume 2. Myopsid and Oegopsid Squids. FAO Species Catalogue for Fishery Purposes No. 4, Vol. 2. FAO, Rome. pp. 148–178.
- Roper, C.F.E. & P. Jereb (2010d). Family Lepidoteuthidae. In: P. Jereb & C.F.E. Roper (eds.) Cephalopods of the world. An annotated and illustrated catalogue of species known to date. Volume 2. Myopsid and Oegopsid Squids. FAO Species Catalogue for Fishery Purposes No. 4, Vol. 2. FAO, Rome. pp. 239–240.
- Roper, C.F.E. & P. Jereb (2010e). Family Magnapinnidae. In: P. Jereb & C.F.E. Roper (eds.) Cephalopods of the world. An annotated and illustrated catalogue of species known to date. Volume 2. Myopsid and Oegopsid Squids. FAO Species Catalogue for Fishery Purposes No. 4, Vol. 2. FAO, Rome. pp. 247–249.
- Roper, C.F.E. & P. Jereb (2010f). Family Mastigoteuthidae. In: P. Jereb & C.F.E. Roper (eds.) Cephalopods of the world. An annotated and illustrated catalogue of species known to date. Volume 2. Myopsid and Oegopsid Squids. FAO Species Catalogue for Fishery Purposes No. 4, Vol. 2. FAO, Rome. pp. 250–256.
- Roper, C.F.E. & P. Jereb (2010g). Family Octopoteuthidae. In: P. Jereb & C.F.E. Roper (eds.) Cephalopods of the world. An annotated and illustrated catalogue of species known to date. Volume 2. Myopsid and Oegopsid Squids. FAO Species Catalogue for Fishery Purposes No. 4, Vol. 2. FAO, Rome. pp. 262–268.
- Roper, C.F.E. & P. Jereb (2010h). Family Onychoteuthidae. In: P. Jereb & C.F.E. Roper (eds.) Cephalopods of the world. An annotated and illustrated catalogue of species known to date. Volume 2. Myopsid and Oegopsid Squids. FAO Species Catalogue for Fishery Purposes No. 4, Vol. 2. FAO, Rome. pp. 348–369.
- Roper, C.F.E. & P. Jereb (2010i). Family Pholidoteuthidae. In: P. Jereb & C.F.E. Roper (eds.) Cephalopods of the world. An annotated and illustrated catalogue of species known to date. Volume 2. Myopsid and Oegopsid Squids. FAO Species Catalogue for Fishery Purposes No. 4, Vol. 2. FAO, Rome. pp. 370–373.
- Roper, C.F.E. & P. Jereb (2010j). Family Thysanoteuthidae. In: P. Jereb & C.F.E. Roper (eds.) Cephalopods of the world. An annotated and illustrated catalogue of species known to date. Volume 2. Myopsid and Oegopsid Squids. FAO Species Catalogue for Fishery Purposes No. 4, Vol. 2. FAO, Rome. pp. 384–387.
- Roper, C.F.E., C. Nigmatullin & P. Jereb (2010). Family Ommastrephidae. In: P. Jereb & C.F.E. Roper (eds.) Cephalopods of the world. An annotated and illustrated catalogue of species known to date. Volume 2. Myopsid and Oegopsid Squids. FAO Species Catalogue for Fishery Purposes No. 4, Vol. 2. FAO, Rome. pp. 269–347.
- Roper, C.F.E. & E.K. Shea (2013). Unanswered questions about the giant squid Architeuthis (Architeuthidae) illustrate our incomplete knowledge of coleoid cephalopods. American Malacological Bulletin 31(1): 109–122. doi:10.4003/006.031.0104
- Roper C.F.E., M.J. Sweeney & C.E. Nauen (1984). Cephalopods of the world. Food and Agriculture Organization, Rome, Italy.
- Roper, C.F.E. & M. Vecchione (1993). A geographic and taxonomic review of Taningia danae Joubin, 1931 (Cephalopoda: Octopoteuthidae), with new records and observations on bioluminescence. In: T. Okutani, R.K. O'Dor & T. Kubodera (eds.) Recent Advances in Cephalopod Fisheries Biology. Tokai University Press, Tokyo. pp. 441–456.
- Roper, C.F.E. & G.L. Voss (1983). Guidelines for taxonomic descriptions of cephalopod species. Memoirs of the National Museum of Victoria 44: 48–63.
- Roper, C.F.E. & R.E. Young (1972). First records of juvenile giant squid, Architeuthis (Cephalopoda: Oegopsida). Proceedings of the Biological Society of Washington 85(16): 205–222.
- Rosewater, J.R. (1965). The family Tridacnidae in the Indo-Pacific. Indo-Pacific Mollusca 1(6): 347–408.
- Salcedo-Vargas, M.A. (1999). An asperoteuthid squid (Mollusca: Cephalopoda: Chiroteuthidae) from New Zealand misidentified as Architeuthis. Mitteilungen aus dem Museum für Naturkunde in Berlin, Zoologische Reihe 75(1): 47–49. doi:10.1002/mmnz.19990750106
- Sanderson, I.T. (1956). Follow the Whale. Little, Brown and Co., Boston. 423 pp.
- Santos, M.B., G.J. Pierce, Á.F. González, F. Santos, M.A. Vázquez, M.A. Santos & M.A. Collins (2001). First records of Taningia danae (Cephalopoda: Octopoteuthidae) in Galician waters (north-west Spain) and in Scottish waters (UK). Journal of the Marine Biological Association of the UK 81(2): 355–356. doi:10.1017/S0025315401003903
- Scheel, D. & R. Anderson (2012). Variability in the diet specialization of Enteroctopus dofleini (Cephalopoda: Octopodidae) in the eastern Pacific examined from midden contents. American Malacological Bulletin 30(2): 267–279. doi:10.4003/006.030.0206
- Schlegelmilch, R. (1998). Die Belemniten des süddeutschen Jura: Ein Bestimmungsbuch für Geowissenschaftler und Fossiliensammler. Gustav Fisher, Stuttgart. ISBN 3437255266. doi:10.1007/978-3-8274-3083-0 (German)
- Schmitz, L., R. Motani, C.E Oufiero, C.H. Martin, M.D. McGee, A.R. Gamarra, J.J. Lee & P.C. Wainwright (2013). Allometry indicates giant eyes of giant squid are not exceptional. BMC Evolutionary Biology 13: 45. doi:10.1186/1471-2148-13-45
- Scott, G. & M.H. Moore (1928). Ammonites of enormous size from the Texas Cretaceous. Journal of Paleontology 2(4): 273–279. JSTOR 1298015
- Scully, T. (2008). Neuroscience: The great squid hunt. Nature 454(7207): 934–936. doi:10.1038/454934a
- Starkey, J.D. (1963). I saw a sea monster. Animals 2(23): 629, 644.
- Staub, F. (1993). Requin bleu, calmar géant et cachalot. Proceedings of the Royal Society of Arts and Sciences of Mauritius 5(3): 141–145.
- Stevens, G.R. (1985). A revision of the Lytoceratinae (Subclass Ammonoidea) including Lytoceras taharoaense n. sp., Upper Jurassic, New Zealand. New Zealand Journal of Geology and Geophysics 28(1): 153–185. doi:10.1080/00288306.1985.10422282
- Stevens, G.R. (1988). Giant ammonites: a review. In: J. Wiedmann & J. Kullmann (eds.) Cephalopods – Present and Past: O.H. Schindewolf Symposium Tübingen 1985. E. Schweizerbart'sche Verlagsbuchhandlung, Stuttgart. pp. 141–166.
- Summesberger, H. (1979). Eine obersantone Ammonitenfauna aus dem Becken von Gosau (Oberösterreich). Annalen des Naturhistorischen Museums Wien 82: 109–176. (German)
- Sweeney, M.J. & C.F.E. Roper (2001). Records of Architeuthis Specimens from Published Reports. National Museum of Natural History, Smithsonian Institution. 132 pp.
- Tanabe, K., Y. Hikida & Y. Iba (2006). Two coleoid jaws from the Upper Cretaceous of Hokkaido, Japan. Journal of Paleontology 80(1): 138–145. doi:10.1666/0022-3360(2006)080[0138:TCJFTU]2.0.CO;2
- Teichert, C. (1927). Der estländische Glint. Natur und Museum 57(6): 264–272. (German)
- Teichert, C. & B. Kummel (1960). Size of endoceroid cephalopods. Breviora Museum of Comparative Zoology 128: 1–7.
- Tsuchiya, K. & T. Okutani (1993). Rare and interesting squids in Japan -X. Recent occurrences of big squids from Okinawa. Venus 52: 299-311.
- Vecchione, M., T. Kubodera & R.E. Young (2010). Taningia danae Joubin 1931. Tree of Life Web Project.
- Vecchione, M., K.M. Mangold & R.E. Young (2008). Cirrata Grimpe, 1916. Tree of Life Web Project.
- Vecchione, M., R.E. Young, Á. Guerra, D.J. Lindsay, D.A. Clague, J.M. Bernhard, W.W. Sager, Á.F. González, F.J. Rocha & M. Segonzac (2001a). Worldwide observations of remarkable deep-sea squids. Science 294(5551): 2505–2506. doi:10.1126/science.294.5551.2505
- Vecchione, M., R.E. Young, Á. Guerra, D.J. Lindsay, D.A. Clague, J.M. Bernhard, W.W. Sager, Á.F. González, F.J. Rocha & M. Segonzac (2001b). Cephalopods in Action. Smithsonian National Museum of Natural History.
- Vecchione, M., R.E. Young & K. Tsuchiya (2011). Onykia (Onykia) robsoni (Adam, 1962). Tree of Life Web Project.
- Verrill, A.E. (1875a). The colossal cephalopods of the North Atlantic. American Naturalist 9(1): 21–36. JSTOR 2448361
- Verrill, A.E. (1875b). The colossal cephalopods of the North Atlantic, II. American Naturalist 9(2): 78–86. JSTOR 2448586
- Verrill, A.E. (1876). Note on gigantic cephalopods,—a correction. The American Journal of Science and Arts 12(3): 236–237.
- Verrill, A.E. (1880). The cephalopods of the north-eastern coast of America. Part I. The gigantic squids (Architeuthis) and their allies; with observations on similar large species from foreign localities. Transactions of the Connecticut Academy of Sciences 5(5): 177–257. doi:10.5962/bhl.title.35965
- Walker, M. (2010). Super squid sex organ discovered. BBC Earth News, 7 July 2010.
- Weis, R. & N. Mariotti (2007). A belemnite fauna from the Aalenian-Bajocian boundary beds of the Grand Duchy of Luxembourg (NE Paris Basin). Bollettino della Società Paleontologica Italiana 46(2–3): 149–174.
- Westermann, G.E.G. (1966). The holotype (plastotype) of ?Titanites occidentalis Frebold from the Kootenay sandstone (Upper Jurassic) of southern British Columbia. Canadian Journal of Earth Sciences 3(5): 623–625. doi:10.1139/e66-043
- Winkelmann, I., P.F. Campos, J. Strugnell, Y. Cherel, P.J. Smith, T. Kubodera, L. Allcock, M.-L. Kampmann, H. Schroeder, Á. Guerra, M. Norman, J. Finn, D. Ingrao, M. Clarke & M.T.P. Gilbert (2013). Mitochondrial genome diversity and population structure of the giant squid Architeuthis: genetics sheds new light on one of the most enigmatic marine species. Proceedings of the Royal Society B: Biological Sciences 280(1759): 20130273. doi:10.1098/rspb.2013.0273
- Wolff, G.A. (1981). A beak key for eight eastern tropical Pacific cephalopod species with relationships between their beak dimensions and size. Fishery Bulletin 80(2): 357–370.
- Wolff, G.A. (1984). Identification and estimation of size from the beaks of 18 species of cephalopods from the Pacific Ocean. NOAA Technical Report NMFS 17, NOAA/National Marine Fisheries Service.
- Wong, K. (2000). Supersized Squid. Scientific American, 8 November 2000.
- Wood, G.L. (1982). The Guinness Book of Animal Facts and Feats. Guinness Superlatives, London. 252 pp. ISBN 0-85112-235-3.
- Wood, J.B. & R.K. O'Dor (2000). Do larger cephalopods live longer? Effects of temperature and phylogeny on interspecific comparisons of age and size at maturity. Marine Biology 136(1): 91–99. doi:10.1007/s002270050012
- Wormuth, J.H. (1976). The biogeography and numerical taxonomy of the oegopsid squid family Ommastrephidae in the Pacific Ocean. Bulletin of the Scripps Institution of Oceanography 23: 1–96.
- Wright, C.W. & W.J. Kennedy (1980). Origin, evolution and systematics of the dwarf acanthoceratid Protacanthoceras Spath, 1923 (Cretaceous Ammonoidea). Bulletin of the British Museum (Natural History): Geology 34(2): 65–107.
- Yasuda, T. (2004). 日本近海における巨大エチゼンクラゲ Nemopilema nomurai の大発生について. [On the unusual occurrence of the giant medusa, Nemopilema nomurai in Japanese waters.] Bulletin of the Japanese Society of Scientific Fisheries 70(3): 380–386. NAID 110003145750 (Japanese)
- Young, R.E. & K.M. Mangold (2010). Megalocranchia Pfeffer, 1884. Tree of Life Web Project.
- Young, R.E., C.F.E. Roper & M. Vecchione (2015). Heteroteuthinae Appellof, 1898. Tree of Life Web Project.
- Yukhov, V.L. (1974). The discovery of giant squids. Priroda [1974](6): 60–63. (Russian)
- Zeidler, W. (1981). A giant deep-sea squid, Taningia sp., from South Australian waters. Transactions of the Royal Society of South Australia 105(4): 218.
- Zuyev, G., C. Nigmatullin, M. Chesalin & K. Nesis (2002). Main results of long-term worldwide studies on tropical nektonic oceanic squid genus Sthenoteuthis: an overview of the Soviet investigations. Bulletin of Marine Science 71(2): 1019–1060.
Further reading
- Ammonites and other fossil cephalopods
- Dommergues, J.-L., S. Montuire & P. Neige (2002). Size patterns through time: the case of the Early Jurassic ammonite radiation. Paleobiology 28(4): 423–434. JSTOR 3595493
- Hallam, A. (1975). Evolutionary size increase and longevity in Jurassic bivalves and ammonites. Nature 258: 493–496. doi:10.1038/258493a0
- Hewitt, R.A. & J.M. Hurst (1977). Size changes in Jurassic liparoceratid ammonites and their stratigraphical and ecological significance. Lethaia 10(4): 287–301. doi:10.1111/j.1502-3931.1977.tb00623.x
- Kennedy, W.J. (1977). [Ammonite evolution:] Evolutionary size changes. In: A. Hallam (ed.) Patterns of evolution, as illustrated by the fossil record. Elsevier Scientific Publishing Company, Amsterdam. pp. 288–290.
- Kummel, B. (1948). Environmental significance of dwarfed cephalopods. Journal of Sedimentary Petrology 18(2): 61–64. doi:10.1306/D4269305-2B26-11D7-8648000102C1865D
- Landman, N.H., S.M. Klofak & K.B. Sarg (2003). Variation in adult size of scaphitid ammonites from the Upper Cretaceous Pierre Shale and Fox Hills Formation. In: P.J. Harries (ed.) High-Resolution Approaches in Stratigraphic Paleontology. Springer. pp. 149–194. doi:10.1007/978-1-4020-9053-0_5
- Manger, W.L., L.K. Meeks & D.A. Stephen (1999). Pathologic gigantism in middle Carboniferous cephalopods, southern midcontinent, United States. In: F. Olóriz & F.J. Rodríguez-Tovar (eds.) Advancing Research on Living and Fossil Cephalopods. Kluwer Academic/Plenum Publishers, New York. pp. 77–89.
- Mignot, Y., S. Elmi & J.-L. Dommergues (1993). Croissance et miniaturisation de quelques Hildoceras (Cephalopoda) en liaison avec des environnements contraignants de la Téthys toarcienne. Geobios 26(Supplement 1): 305–312. doi:10.1016/S0016-6995(06)80384-0 (French)
- Newell, N.D. (1949). Phyletic size increase, an important trend illustrated by fossil invertebrates. Evolution 3(2): 103–124. JSTOR 2405545
- Reboulet, S. (2001). Limiting factors on shell growth, mode of life and segregation of valanginian ammonoid populations: evidence from adult-size variations. Geobios 34(4): 423–435. doi:10.1016/S0016-6995(01)80006-1
- Living cephalopods
- Boletzky, S.v. (1997). Developmental constraints and heterochrony: a new look at offspring size in cephalopod molluscs. Geobios 30(Supplement 2): 267–275. doi:10.1016/S0016-6995(97)80102-7
- Boletzky, S.v. (2002). How small is "very small" in coleoid cephalopods? Berliner Paläobiologische Abhandlungen 1: 14–15.
- Haaker, P.L. (1985). Observations of a dwarf octopus, Octopus micropyrsus. Shells and Sea Life 17(1): 39–40.
- Jackson, G.D. & M.L. Domeier (2003). The effects of an extraordinary El Niño / La Niña event on the size and growth of the squid Loligo opalescens off Southern California. Marine Biology 142(5): 925–935. doi:10.1007/s00227-002-1005-4
- Pecl, G.T., M.A. Steer & K.E. Hodgson (2004). The role of hatchling size in generating the intrinsic size-at-age variability of cephalopods: extending the Forsythe Hypothesis. Marine and Freshwater Research 55(4): 387–394. doi:10.1071/MF03153
External links
|
|
|
