Coronary stent
| Coronary stent | |
|---|---|
| Intervention | |
 An example of a coronary stent. This Taxus stent is labeled as a drug-eluting stent. | |
| ICD-9-CM | 36.06 |
A coronary stent is a tube-shaped device placed in the coronary arteries that supply blood to the heart, to keep the arteries open in the treatment of coronary heart disease. It is used in a procedure called percutaneous coronary intervention (PCI). Stents reduce chest pain and have been shown to improve survivability in the event of an acute myocardial infarction.[1]
Similar stents and procedures are used in non-coronary vessels e.g. in the legs in peripheral artery disease.
Medical uses
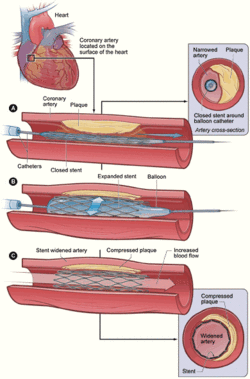

Treating a blocked ("stenosed") coronary artery with a stent follows the same steps as other angioplasty procedures with a few important differences. The interventional cardiologist uses angiography to assess the location and estimate the size of the blockage ("lesion") by injecting a contrast medium through the guide catheter and viewing the flow of blood through the downstream coronary arteries. Intravascular ultrasound (IVUS) may be used to assess the lesion's thickness and hardness ("calcification"). The cardiologist uses this information to decide whether to treat the lesion with a stent, and if so, what kind and size. Drug eluting stents are most often sold as a unit, with the stent in its collapsed form attached onto the outside of a balloon catheter. Outside the US, physicians may perform "direct stenting" where the stent is threaded through the lesion and expanded. Common practice in the US is to predilate the blockage before delivering the stent. Predilation is accomplished by threading the lesion with an ordinary balloon catheter and expanding it to the vessel's original diameter. The physician withdraws this catheter and threads the stent on its balloon catheter through the lesion. The physician expands the balloon which deforms the metal stent to its expanded size. The cardiologist may "customize" the fit of the stent to match the blood vessel's shape, using IVUS to guide the work.[2] It is critically important that the framework of the stent be in direct contact with the walls of the vessel to minimize potential complications such as blood clot formation. Very long lesions may require more than one stent—this result of this treatment is sometimes referred to as a "full metal jacket".[3]
The procedure itself is performed in a catheterization clinic ("cath lab"). Barring complications, patients undergoing catheterizations are kept at least overnight for observation.[4]
Dealing with lesions near branches in the coronary arteries presents additional challenges and requires additional techniques.[5]
Risks
Re-occlusion
Coronary artery stents, typically a metal framework, can be placed inside the artery to help keep it open. However, as the stent is a foreign object (not native to the body), it incites an immune response. This may cause scar tissue (cell proliferation) to rapidly grow over the stent. In addition, there is a strong tendency for clots to form at the site where the stent damages the arterial wall. Since platelets are involved in the clotting process, patients must take dual antiplatelet therapy afterwards, usually clopidogrel or Brilinta and aspirin for one year and aspirin indefinitely.[6] In order to reduce the treatment, a new generation of stent has been developed with biodegradable polymer.
However, the dual antiplatelet therapy may be insufficient to fully prevent clots that may result in stent thrombosis; these and the cell proliferation may cause the standard (“bare-metal”) stents to become blocked (restenosis). Drug-eluting stents were designed to lessen this problem; by releasing an antiproliferative drug (drugs typically used against cancer or as immunosuppressants), they can help avoid this in-stent restenosis (re-narrowing).
Restenosis
One of the drawbacks of vascular stents is the potential for restenosis via the development of a thick smooth muscle tissue inside the lumen, the so-called neointima. Development of a neointima is variable but can at times be so severe as to re-occlude the vessel lumen (restenosis), especially in the case of smaller diameter vessels, which often results in reintervention. Consequently, current research focuses on the reduction of neointima after stent placement. Substantial improvements have been made, including the use of more biocompatible materials, anti-inflammatory drug-eluting stents, resorbable stents, and others. Restenosis can be treated with a reintervention using the same method.
On September 4, 2007, an international study showed that some heart attack patients would be better off not using drug-coated stents in emergency to open their clogged arteries (patients were five times as likely to die after two years as those who received bare-metal stents). Dr. Valentin Fuster, director of the Cardiovascular Institute at Mount Sinai School of Medicine in New York City said stents are less commonly used in Europe, implanted in only about 15 percent of patients there, while drug-lined stents are used in up to 30 percent of Americans having heart attacks. The new research was presented by Dr. Gabriel Steg of the Hospital Bichat-Claude Bernard in Paris at a meeting of the European Society of Cardiology in Vienna. Dr. Eckhart Fleck, director of cardiology at the German Heart Institute in Berlin and a spokesman for the European Society of Cardiology, said, "Drug-eluting stents are not for everyone."[7]
Controversy
The value of stenting in rescuing someone having a heart attack (by immediately alleviating an obstruction) is clearly defined in multiple studies, but studies have failed to find reduction in hard endpoints for stents vs. medical therapy in stable angina patients (see below). The artery-opening stent can temporarily alleviate chest pain, but does not contribute to longevity. The "vast majority of heart attacks do not originate with obstructions that narrow arteries."[8]
A more permanent and successful way to prevent heart attacks in patients at high risk is to give up smoking, to exercise regularly, and take "drugs to get blood pressure under control, drive cholesterol levels down and prevent blood clotting".[8]
Some cardiologists believe that stents are overused; however, in certain patient groups, such as the elderly, GRACE and other studies have found evidence of under-use. One cardiologist was convicted of billing patients for performing medically unnecessary stenting.[9][10] Guidelines recommend a stress test before implanting stents, but most patients do not receive a stress test.[11]
Research
While revascularisation (by stenting or bypass surgery) is of clear benefit in reducing mortality and morbidity in patients with acute symptoms (acute coronary syndromes) including myocardial infarction, their benefit is less marked in stable patients. Clinical trials have failed to demonstrate that coronary stents improve survival over best medical treatment.
- The COURAGE trial compared PCI with optimum medical therapy. Of note, the trial excluded a large number of patients at the outset and undertook angiography in all patients at baseline, thus the results only apply to a subset of patients and should not be over-generalised. COURAGE concluded that in patients with stable coronary artery disease PCI did not reduce the death, myocardial infarction or other major cardiac events when added to optimum medical therapy.[12]
- The MASS-II trial compared PCI, CABG and optimum medical therapy for the treatment of multi-vessel coronary artery disease. The MASS-II trial showed no difference in cardiac death or acute MI among patients in the CABG, PCI, or MT group. However, it did show a significantly greater need for additional revascularization procedures in patients who underwent PCI.[13][14]
- The SYNTAX Trial[15] is a manufacturer-funded trial with a primary endpoint of death, cardiovascular events, and myocardial infarction, and also the need for repeat vascularization, in patients with blocked or narrowed arteries. Patients were randomized to either CABG surgery or a drug-eluting stent (the Boston Scientific TAXUS paclitaxel-eluting stent). SYNTAX found the two strategies to be similar for hard endpoints (death and MI). Those receiving PCI required more repeat revascularisation (hence the primary endpoint analysis did not find PCI to be non-inferior), but those undergoing CABG had significantly more strokes pre or perioperatively. Use of the SYNTAX risk score is being investigated as a method of identifying those multivessel disease patients in whom PCI is a reasonable option vs those in whom CABG remains the preferred strategy.
Several other clinical trials have been performed to examine the efficacy of coronary stenting and compare with other treatment options. A consensus of the medical community does not exist.
History
The first type, developed by John Robert Dugan of Shelbyville, Indiana, were bare-metal stents. More recent are drug-eluting stents.
In development are stents with biocompatible surface coatings which do not elute drugs, and also absorbable stents (metal or polymer).
Videos
 Stenting of coarctation of the aorta
Stenting of coarctation of the aorta
_-_Dr._Marcelo_Cerezo_(video_19_de_33).webm.jpg)
References
- ↑ Armstrong P; WEST Steering Committee (2006). "A comparison of pharmacologic therapy with/without timely coronary intervention vs. primary percutaneous intervention early after ST-elevation myocardial infarction: the WEST (Which Early ST-elevation myocardial infarction Therapy) study". Eur Heart J. 27 (10): 1530–1538. doi:10.1093/eurheartj/ehl088. PMID 16757491.
- ↑ Intravascular Ultrasound - Angioplasty.Org
- ↑ Aoki J, Ong ATL, Granillo GAR, McFadden EP, van Mieghem CAG, Valgimigli M, Tsuchida K, Sianos G, Regar E, de Jaegere PPT, van der Giessen WJ, de Feyter PJ, van Domburg RT, Serruys PW (November 2005). ""Full metal jacket" (stented length > or =64 mm) using drug-eluting stents for de novo coronary artery lesions". Am Heart J. 150 (5): 994–9. doi:10.1016/j.ahj.2005.01.050. PMID 16290984.
- ↑ Angioplasty 101 Angioplasty.Org
- ↑ http://indianheartjournal.com/ihj06/mar_apr_06/108-119.html
- ↑ Michel, Thomas (2006) [1941]. "Treatment of Myocardial Ischemia". In Laurence L. Brunton; John S. Lazo; Keith L. Parker. Goodman & Gilman's The Pharmacological Basis of Therapeutics (11th ed.). New York: McGraw-Hill. p. 842.
- ↑ Yahoo.com, International study shows stent risks
- 1 2 Kolata, Gina. "New Heart Studies Question the Value Of Opening Arteries" The New York Times, March 21, 2004. Retrieved January 14, 2011.
- ↑ David Armstrong (24 October 2013). "The Cardiologist Who Spread Heart Disease". Bloomberg.
- ↑ Peter Waldman; David Armstrong & Sydney P. Freedberg (26 September 2013). "Deaths Linked to Cardiac Stents Rise as Overuse Seen". Bloomberg.
- ↑ A simple health care fix fizzles out, Kenneth J. Winstein, Wall Street Journal, Feb. 11, 2010.
- ↑ Boden WE; O'Rourke RA; Teo KK; Hartigan PM; Maron DJ; Kostuk WJ; Knudtson M; Dada M; Casperson P; Harris CL; Chaitman BR; Shaw L; Gosselin G; Nawaz S; Title LM; Gau G; Blaustein AS; Booth DC; Bates ER; Spertus JA; Berman DS; Mancini GB; Weintraub WS; COURAGE Trial Research Group (2007-04-12). "Optimal medical therapy with or without PCI for stable coronary disease". N Engl J Med. 356 (15): 1503–16. doi:10.1056/NEJMoa070829. PMID 17387127.
- ↑ Hueb W, Soares PR, Gersh BJ, César LA, Luz PL, Puig LB, Martinez EM, Oliveira SA, Ramires JA (2004-05-19). "The medicine, angioplasty, or surgery study (MASS-II): a randomized, controlled clinical trial of three therapeutic strategies for multivessel coronary artery disease: one-year results". J Am Coll Cardiol. 43 (10): 1743–51. doi:10.1016/j.jacc.2003.08.065. PMID 15145093.
- ↑ http://circ.ahajournals.org/cgi/content/abstract/circulationaha;115/9/1082 MASS-II 5yr follow-up.
- ↑ Clinical trial number NCT00114972 at ClinicalTrials.gov SYNTAX trial 2005-2008