Glucagon
| View/Edit Human | |||
Glucagon is a peptide hormone, produced by alpha cells of the pancreas. It works to raise the concentration of glucose in the bloodstream. Its effect is opposite to that of insulin, which lowers the glucose.[2]
The pancreas releases glucagon when the concentration of glucose in the bloodstream falls too low. Glucagon causes the liver to convert stored glycogen into glucose, which is released into the bloodstream.[3] High blood-glucose levels stimulate the release of insulin. Insulin allows glucose to be taken up and used by insulin-dependent tissues. Thus, glucagon and insulin are part of a feedback system that keeps blood glucose levels at a stable level. It increases energy expenditure and is elevated under conditions of stress.[4] Glucagon belongs to a family of several other related hormones.
It is on the WHO Model List of Essential Medicines, the most important medications needed in a basic health system.[5]
Function
Glucagon generally elevates the concentration of glucose in the blood by promoting gluconeogenesis and glycogenolysis.
Glucose is stored in the liver in the form of the polysaccharide glycogen, which is a glucan (a polymer made up of glucose molecules). Liver cells (hepatocytes) have glucagon receptors. When glucagon binds to the glucagon receptors, the liver cells convert the glycogen into individual glucose molecules and release them into the bloodstream, in a process known as glycogenolysis. As these stores become depleted, glucagon then encourages the liver and kidney to synthesize additional glucose by gluconeogenesis. Glucagon turns off glycolysis in the liver, causing glycolytic intermediates to be shuttled to gluconeogenesis.
Glucagon also regulates the rate of glucose production through lipolysis. Glucagon induces lipolysis in humans under conditions of insulin suppression (such as diabetes mellitus type 1).[6]
Glucagon production appears to be dependent on the central nervous system through pathways yet to be defined. In invertebrate animals, eyestalk removal has been reported to affect glucagon production. Excising the eyestalk in young crayfish produces glucagon-induced hyperglycemia.[7]
Medical uses
 | |
| Identifiers | |
|---|---|
| PubChem (CID) | 16186314 |
| IUPHAR/BPS | 1136 |
| ChemSpider |
10481928 |
| UNII |
76LA80IG2G |
| ChEMBL |
CHEMBL266481 |
| Chemical and physical data | |
| Formula | C153H225N43O49S |
| Molar mass | 3482.747314 g/mol |
| |
| | |
Hypoglycemia
An injectable form of glucagon is vital first aid in cases of severe hypoglycemia when the victim is unconscious or for other reasons cannot take glucose orally. The dose for an adult is typically 1 milligram, and the glucagon is given by intramuscular, intravenous or subcutaneous injection, and quickly raises blood glucose levels. To use the injectable form, it must be reconstituted prior to use, a step that requires a sterile diluent to be injected into a vial containing powdered glucagon, because the hormone is highly unstable when dissolved in solution. When dissolved in a fluid state, glucagon can form amyloid fibrils, or tightly woven chains of proteins made up of the individual glucagon peptides, and once glucagon begins to fibrilize, it becomes useless when injected, as the glucagon cannot be absorbed and used by the body. The reconstitution process makes using glucagon cumbersome, although there are a number of products now in development from a number of companies that aim to make the product easier to use.
Beta blocker overdose
Anecdotal evidence suggests a benefit of higher doses of glucagon in the treatment of overdose with beta blockers; the likely mechanism of action is the increase of cAMP in the myocardium, in effect bypassing the β-adrenergic second messenger system.[8]
Anaphylaxis
Some people who have anaphylaxis and are on beta blockers are resistant to epinephrine. In this situation glucagon intravenously may be useful to treat their low blood pressure.[9]
Impacted food bolus
Glucagon relaxes the lower esophageal sphincter and may be used in those with an impacted food bolus in the esophagus ("steakhouse syndrome").[10] There is little evidence for glucagon's effectiveness in this condition,[11][12][13] and glucagon may induce nausea and vomiting,[13] but considering the safety of glucagon this is still considered an acceptable option as long it does not lead to delays in arranging other treatments.[14][15]
Endoscopic retrograde cholangiopancreatography
Glucagon's effect of decreasing cAMP causes relaxation of splanchnic smooth muscle, allowing cannulation of the duodenum during the endoscopic retrograde cholangiopancreatography (ERCP) procedure.
Adverse effects
Glucagon acts very quickly; common side-effects include headache and nausea.
Drug interactions: Glucagon interacts only with oral anticoagulants, increasing the tendency to bleed.[16]
Contraindications
While glucagon can be used clinically to treat various forms of hypoglycemia, it is contraindicated in patients with pheochromocytoma, as it can induce the tumor to release catecholamines, leading to a sudden elevation in blood pressure.[17] Likewise, glucagon is contraindicated in patients with an insulinoma, as its hyperglycemic effect can induce the tumor to release insulin, leading to rebound hypoglycemia.[17]
Mechanism of action
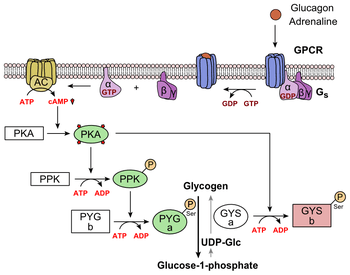
Glucagon binds to the glucagon receptor, a G protein-coupled receptor, located in the plasma membrane. The conformation change in the receptor activates G proteins, a heterotrimeric protein with α, β, and γ subunits. When the G protein interacts with the receptor, it undergoes a conformational change that results in the replacement of the GDP molecule that was bound to the α subunit with a GTP molecule. This substitution results in the releasing of the α subunit from the β and γ subunits. The alpha subunit specifically activates the next enzyme in the cascade, adenylate cyclase.
Adenylate cyclase manufactures cyclic adenosine monophosphate (cyclic AMP or cAMP), which activates protein kinase A (cAMP-dependent protein kinase). This enzyme, in turn, activates phosphorylase kinase, which then phosphorylates glycogen phosphorylase b, converting it into the active form called phosphorylase a. Phosphorylase a is the enzyme responsible for the release of glucose-1-phosphate from glycogen polymers.
Additionally, the coordinated control of glycolysis and gluconeogenesis in the liver is adjusted by the phosphorylation state of the enzymes that catalyze the formation of a potent activator of glycolysis called fructose-2,6-bisphosphate.[18] The enzyme protein kinase A that was stimulated by the cascade initiated by glucagon will also phosphorylate a single serine residue of the bifunctional polypeptide chain containing both the enzymes fructose-2,6-bisphosphatase and phosphofructokinase-2. This covalent phosphorylation initiated by glucagon activates the former and inhibits the latter. This regulates the reaction catalyzing fructose-2,6-bisphosphate (a potent activator of phosphofructokinase-1, the enzyme that is the primary regulatory step of glycolysis)[19] by slowing the rate of its formation, thereby inhibiting the flux of the glycolysis pathway and allowing gluconeogenesis to predominate. This process is reversible in the absence of glucagon (and thus, the presence of insulin).
Glucagon stimulation of PKA also inactivates the glycolytic enzyme pyruvate kinase.[20]
Physiology
Production
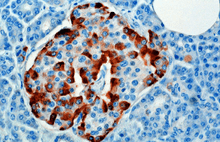
The hormone is synthesized and secreted from alpha cells (α-cells) of the islets of Langerhans, which are located in the endocrine portion of the pancreas. Production, which is otherwise freerunning, is suppressed/regulated by insulin from the adjacent beta cells. When blood sugar drops, insulin production drops and more glucagon is produced.[21] In rodents, the alpha cells are located in the outer rim of the islet. Human islet structure is much less segregated, and alpha cells are distributed throughout the islet in close proximity to beta cells. Glucagon is also produced by alpha cells in the stomach.[22]
Regulation
Secretion of glucagon is stimulated by:
- Hypoglycemia
- Epinephrine (via β2, α2,[23] and α1[24] adrenergic receptors)
- Arginine
- Alanine (often from muscle-derived pyruvate/glutamate transamination (see alanine transaminase reaction).
- Acetylcholine[25]
- Cholecystokinin
Secretion of glucagon is inhibited by:
- Somatostatin
- Insulin (via GABA)[26]
- PPARγ/retinoid X receptor heterodimer.[27]
- Increased free fatty acids and keto acids into the blood.[28]
- Increased urea production
Structure
Glucagon is a 29-amino acid polypeptide. Its primary structure in humans is: NH2-His-Ser-Gln-Gly-Thr-Phe-Thr-Ser-Asp-Tyr-Ser-Lys-Tyr-Leu-Asp-Ser-Arg-Arg-Ala-Gln-Asp-Phe-Val-Gln-Trp-Leu-Met-Asn-Thr-COOH.
The polypeptide has a molecular weight of 3485 daltons. Glucagon is a peptide (nonsteroid) hormone.
Glucagon is generated from the cleavage of proglucagon by proprotein convertase 2 in pancreatic islet α cells. In intestinal L cells, proglucagon is cleaved to the alternate products glicentin, GLP-1 (an incretin), IP-2, and GLP-2 (promotes intestinal growth).[29]
Pathology
Abnormally elevated levels of glucagon may be caused by pancreatic tumors, such as glucagonoma, symptoms of which include necrolytic migratory erythema, reduced amino acids, and hyperglycemia. It may occur alone or in the context of multiple endocrine neoplasia type 1.
History
In the 1920s, Kimball and Murlin studied pancreatic extracts, and found an additional substance with hyperglycemic properties. They described glucagon in 1923.[30] The amino acid sequence of glucagon was described in the late 1950s.[31] A more complete understanding of its role in physiology and disease was not established until the 1970s, when a specific radioimmunoassay was developed.
Etymology
Glucagon was named in 1923, probably from the Greek γλυκός sweet, and ἄγειν to lead.[32]
See also
- Cortisol
- Diabetes mellitus
- Glucagon-like peptide-1
- Glucagon-like peptide-2
- Insulin
- Islets of Langerhans
- Pancreas
- Proglucagon
- Tyrosine kinase
References
- ↑ "Human PubMed Reference:".
- ↑ Reece J, Campbell N (2002). Biology. San Francisco: Benjamin Cummings. ISBN 0-8053-6624-5.
- ↑ Orsay, Jonathan (2014). Biology 1: Molecules. Examkrackers Inc. p. 77. ISBN 978-1-893858-70-1.
- ↑ Jones, BJ; Tan, T; Bloom, SR (March 2012). "Minireview: Glucagon in stress and energy homeostasis.". Endocrinology. 153 (3): 1049–54. doi:10.1210/en.2011-1979. PMC 3281544
 . PMID 22294753.
. PMID 22294753. - ↑ "19th WHO Model List of Essential Medicines (April 2015)" (PDF). WHO. April 2015. Retrieved May 10, 2015.
- ↑ Liljenquist JE, Bomboy JD, Lewis SB, Sinclair-Smith BC, Felts PW, Lacy WW, Crofford OB, Liddle GW (Jan 1974). "Effects of glucagon on lipolysis and ketogenesis in normal and diabetic men" (PDF). The Journal of Clinical Investigation. 53 (1): 190–7. doi:10.1172/JCI107537. PMC 301453
 . PMID 4808635.
. PMID 4808635. - ↑ Leinen RL, Giannini AJ (1983). "Effect of eyestalk removal on glucagon induced hyperglycemia in crayfish". Society for Neuroscience Abstracts. 9: 604.
- ↑ White CM (May 1999). "A review of potential cardiovascular uses of intravenous glucagon administration". Journal of Clinical Pharmacology. 39 (5): 442–7. PMID 10234590.
- ↑ Tang AW (Oct 2003). "A practical guide to anaphylaxis". American Family Physician. 68 (7): 1325–32. PMID 14567487.
- ↑ Ko HH, Enns R (Oct 2008). "Review of food bolus management". Canadian Journal of Gastroenterology = Journal Canadien De Gastroenterologie. 22 (10): 805–8. PMC 2661297
 . PMID 18925301.
. PMID 18925301. - ↑ Arora S, Galich P (Mar 2009). "Myth: glucagon is an effective first-line therapy for esophageal foreign body impaction". Cjem. 11 (2): 169–71. PMID 19272219.
- ↑ Leopard D, Fishpool S, Winter S (Sep 2011). "The management of oesophageal soft food bolus obstruction: a systematic review". Annals of the Royal College of Surgeons of England. 93 (6): 441–4. doi:10.1308/003588411X588090. PMC 3369328
 . PMID 21929913.
. PMID 21929913. - 1 2 Weant KA, Weant MP (Apr 2012). "Safety and efficacy of glucagon for the relief of acute esophageal food impaction". American Journal of Health-System Pharmacy. 69 (7): 573–7. doi:10.2146/ajhp100587. PMID 22441787.
- ↑ Ikenberry SO, Jue TL, Anderson MA, Appalaneni V, Banerjee S, Ben-Menachem T, Decker GA, Fanelli RD, Fisher LR, Fukami N, Harrison ME, Jain R, Khan KM, Krinsky ML, Maple JT, Sharaf R, Strohmeyer L, Dominitz JA (Jun 2011). "Management of ingested foreign bodies and food impactions" (PDF). Gastrointestinal Endoscopy. 73 (6): 1085–1091. doi:10.1016/j.gie.2010.11.010. PMID 21628009.
- ↑ Chauvin A, Viala J, Marteau P, Hermann P, Dray X (Jul 2013). "Management and endoscopic techniques for digestive foreign body and food bolus impaction". Digestive and Liver Disease. 45 (7): 529–42. doi:10.1016/j.dld.2012.11.002. PMID 23266207.
- ↑ Koch-Weser J (Mar 1970). "Potentiation by glucagon of the hypoprothrombinemic action of warfarin". Annals of Internal Medicine. 72 (3): 331–5. doi:10.7326/0003-4819-72-3-331. PMID 5415418.
- 1 2 "Information for the Physician: Glucagon for Injection (rDNA origin)" (PDF). Eli Lilly and Company. Retrieved 2016-10-12.
- ↑ Hue L, Rider MH (Jul 1987). "Role of fructose 2,6-bisphosphate in the control of glycolysis in mammalian tissues". The Biochemical Journal. 245 (2): 313–24. PMC 1148124
 . PMID 2822019.
. PMID 2822019. - ↑ Claus TH, El-Maghrabi MR, Regen DM, Stewart HB, McGrane M, Kountz PD, Nyfeler F, Pilkis J, Pilkis SJ (1984). "The role of fructose 2,6-bisphosphate in the regulation of carbohydrate metabolism". Current Topics in Cellular Regulation. 23: 57–86. doi:10.1016/b978-0-12-152823-2.50006-4. PMID 6327193.
- ↑ Feliú JE, Hue L, Hers HG (Aug 1976). "Hormonal control of pyruvate kinase activity and of gluconeogenesis in isolated hepatocytes". Proceedings of the National Academy of Sciences of the United States of America. 73 (8): 2762–6. doi:10.1073/pnas.73.8.2762. PMC 430732
 . PMID 183209.
. PMID 183209. - ↑ Unger, Roger (January 3, 2012). "Glucagonocentric restructuring of diabetes: a pathophysiologic and therapeutic makeover". NCBI. J Clin Invest. doi:10.1172/JCI60016. Retrieved 10 February 2016.
- ↑ Unger RH, Cherrington AD (2012). "Glucagonocentric restructuring of diabetes: a pathophysiologic and therapeutic makeover". The Journal of Clinical Investigation. 122 (1): 4–12. doi:10.1172/JCI60016. PMC 3248306
 . PMID 22214853.
. PMID 22214853. - ↑ Layden BT, Durai V, Lowe WL (2010). "G-Protein-Coupled Receptors, Pancreatic Islets, and Diabetes". Nature Education. 3 (9): 13.
- ↑ Skoglund G, Lundquist I, Ahrén B (Nov 1987). "Alpha 1- and alpha 2-adrenoceptor activation increases plasma glucagon levels in the mouse". European Journal of Pharmacology. 143 (1): 83–8. doi:10.1016/0014-2999(87)90737-0. PMID 2891547.
- ↑ Honey RN, Weir GC (Oct 1980). "Acetylcholine stimulates insulin, glucagon, and somatostatin release in the perfused chicken pancreas". Endocrinology. 107 (4): 1065–8. doi:10.1210/endo-107-4-1065. PMID 6105951.
- ↑ Xu E, Kumar M, Zhang Y, Ju W, Obata T, Zhang N, Liu S, Wendt A, Deng S, Ebina Y, Wheeler MB, Braun M, Wang Q (Jan 2006). "Intra-islet insulin suppresses glucagon release via GABA-GABAA receptor system". Cell Metabolism. 3 (1): 47–58. doi:10.1016/j.cmet.2005.11.015. PMID 16399504.
- ↑ Krätzner R, Fröhlich F, Lepler K, Schröder M, Röher K, Dickel C, Tzvetkov MV, Quentin T, Oetjen E, Knepel W (Feb 2008). "A peroxisome proliferator-activated receptor gamma-retinoid X receptor heterodimer physically interacts with the transcriptional activator PAX6 to inhibit glucagon gene transcription". Molecular Pharmacology. 73 (2): 509–517. doi:10.1124/mol.107.035568. PMID 17962386.
- ↑ Leonard R. Johnson (2003). Essential Medical Physiology. Academic Press. pp. 643–. ISBN 978-0-12-387584-6.
- ↑ Orskov C, Holst JJ, Poulsen SS, Kirkegaard P (Nov 1987). "Pancreatic and intestinal processing of proglucagon in man". Diabetologia. 30 (11): 874–81. doi:10.1007/BF00274797. PMID 3446554.
- ↑ Kimball C, Murlin J (1923). "Aqueous extracts of pancreas III. Some precipitation reactions of insulin". J. Biol. Chem. 58 (1): 337–348.
- ↑ Bromer W, Winn L, Behrens O (1957). "The amino acid sequence of glucagon V. Location of amide groups, acid degradation studies and summary of sequential evidence". J. Am. Chem. Soc. 79 (11): 2807–2810. doi:10.1021/ja01568a038.
- ↑ glucagon on dictionary.com