Isobaric process
| Thermodynamics | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
The classical Carnot heat engine | ||||||||||||
|
Branches |
||||||||||||
|
||||||||||||
| Book:Thermodynamics | ||||||||||||
An isobaric process is a thermodynamic process in which the pressure stays constant: ΔP = 0. The term derives from the Greek iso- (equal) and baros (weight). The heat transferred to the system does work, but also changes the internal energy of the system. This article uses the chemistry sign convention for work, where positive work is work done on the system. Using this convention, by the first law of thermodynamics,
where W is work, U is internal energy, and Q is heat.[1] Pressure-volume work by the closed system is defined as:
where Δ means change over the whole process, whereas d denotes a differential. Since pressure is constant, this means that
- .
Applying the ideal gas law, this becomes
assuming that the quantity of gas stays constant, e.g., there is no phase transition during a chemical reaction. According to the equipartition theorem, the change in internal energy is related to the temperature of the system by
- ,
where cV is specific heat at a constant volume.
Substituting the last two equations into the first equation produces:
- ,
where cP is specific heat at a constant pressure.
Specific heat capacity
To find the molar specific heat capacity of the gas involved, the following equations apply for any general gas that is calorically perfect. The property γ is either called the adiabatic index or the heat capacity ratio. Some published sources might use k instead of γ.
Molar isochoric specific heat:
- .
Molar isobaric specific heat:
- .
The values for γ are γ = 7/5 for diatomic gases like air and its major components, and γ = 5/3 for monatomic gases like the noble gases. The formulas for specific heats would reduce in these special cases:
Monatomic:
- and
Diatomic:
- and
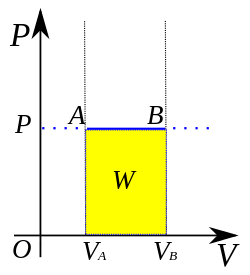
An isobaric process is shown on a P–V diagram as a straight horizontal line, connecting the initial and final thermostatic states. If the process moves towards the right, then it is an expansion. If the process moves towards the left, then it is a compression.
Sign convention for work
The motivation for the specific sign conventions of thermodynamics comes from early development of heat engines. When designing a heat engine, the goal is to have the system produce and deliver work output. The source of energy in a heat engine, is a heat input.
If the volume compresses (ΔV = final volume − initial volume < 0), then W < 0. That is, during isobaric compression the gas does negative work, or the environment does positive work. Restated, the environment does positive work on the gas.
If the volume expands (ΔV = final volume − initial volume > 0), then W > 0. That is, during isobaric expansion the gas does positive work, or equivalently, the environment does negative work. Restated, the gas does positive work on the environment.
If heat is added to the system, then Q > 0. That is, during isobaric expansion/heating, positive heat is added to the gas, or equivalently, the environment receives negative heat. Restated, the gas receives positive heat from the environment.
If the system rejects heat, then Q < 0. That is, during isobaric compression/cooling, negative heat is added to the gas, or equivalently, the environment receives positive heat. Restated, the environment receives positive heat from the gas.
Defining enthalpy
An isochoric process is described by the equation Q = ΔU. It would be convenient to have a similar equation for isobaric processes. Substituting the second equation into the first yields
The quantity U + pV is a state function so that it can be given a name. It is called enthalpy, and is denoted as H. Therefore, an isobaric process can be more succinctly described as
- .
Enthalpy and isochoric specific heat capacity are very useful mathematical constructs, since when analyzing a process in an open system, the situation of zero work occurs when the fluid flows at constant pressure. In an open system, enthalpy is the quantity which is useful to use to keep track of energy content of the fluid.
Variable density viewpoint
A given quantity (mass m) of gas in a changing volume produces a change in density ρ. In this context the ideal gas law is written
where T is thermodynamic temperature and M is molar mass. When R and M are taken as constant, then pressure P can stay constant as the density-temperature quadrant (ρ,T) undergoes a squeeze mapping.[2]
See also
- Adiabatic process
- Cyclic process
- Isochoric process
- Isothermal process
- Polytropic process
- Isenthalpic process
References
- ↑ https://www.grc.nasa.gov/www/k-12/airplane/thermo1.html. Missing or empty
|title=(help) - ↑ Olver, Peter (1999). Classical Invariant Theory. p. 217.
