Norelgestromin
 | |
| Clinical data | |
|---|---|
| Trade names | Evra, Ortho Evra |
| AHFS/Drugs.com | International Drug Names |
| MedlinePlus | a602006 |
| Routes of administration | Transdermal patch |
| ATC code | G03AA13 (WHO) (only combinations with estrogens) |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| Synonyms | 17-Deacetylnorgestimate, levonorgestrel 3-oxime |
| CAS Number |
53016-31-2 |
| PubChem (CID) | 62930 |
| DrugBank |
DB06713 |
| ChemSpider |
56648 |
| UNII |
R0TAY3X631 |
| KEGG |
D05205 |
| ChEBI |
CHEBI:111762 |
| ChEMBL |
CHEMBL1200807 |
| Chemical and physical data | |
| Formula | C21H29NO2 |
| Molar mass | 327.461 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
| | |
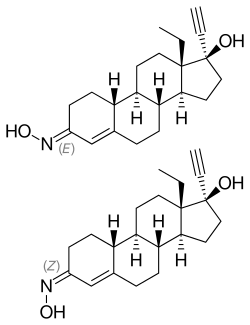
Norelgestromin (INN, USAN, BAN), or norelgestromine, also known as 17-deacetylnorgestimate or norgestrel 3-oxime,[1] is a steroidal progestin of the 19-nortestosterone group used in the contraceptive patch called Evra or Ortho Evra, where it is combined with the estrogen ethinyl estradiol.[2][3] It is one of the active metabolites of norgestimate.[4][5] Unlike many other 19-nortestosterone derivatives, norelgestromin has negligible androgenic activity.[5] The drug was introduced in 2002.[6]
References
- ↑ IARC Working Group on the Evaluation of Carcinogenic Risks to Humans; World Health Organization; International Agency for Research on Cancer (2007). Combined Estrogen-progestogen Contraceptives and Combined Estrogen-progestogen Menopausal Therapy. World Health Organization. pp. 150–151. ISBN 978-92-832-1291-1.
- ↑ Drugs.com: Norelgestromin/Ethinyl Estradiol Patch
- ↑ Crosignani, Pier Giorgio; Nappi, Carmine; Ronsini, Salvatore; Bruni, Vincenzina; Marelli, Silvia; Sonnino, Davide (2009). "Satisfaction and compliance in hormonal contraception: the result of a multicentre clinical study on women's experience with the ethinylestradiol/norelgestromin contraceptive patch in Italy". BMC Women's Health. 9 (1). doi:10.1186/1472-6874-9-18. ISSN 1472-6874.
- ↑ Annette M. Doherty (2003). Annual Reports in Medicinal Chemistry. Academic Press. pp. 362–. ISBN 978-0-12-040538-1.
- 1 2 Stefan Offermanns; Walter Rosenthal (14 August 2008). Encyclopedia of Molecular Pharmacology. Springer Science & Business Media. pp. 391–. ISBN 978-3-540-38916-3.
- ↑ John E. Macor (2012). Annual Reports in Medicinal Chemistry. Academic Press. pp. 620–. ISBN 978-0-12-396492-2.
This article is issued from Wikipedia - version of the 12/2/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.