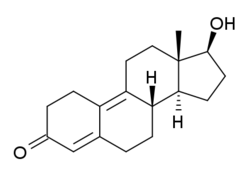
Dienolone
 | |
| Clinical data | |
|---|---|
| ATC code | None |
| Identifiers | |
| |
| Synonyms | RU-3118; Nordienolone |
| CAS Number | 6218-29-7 |
| PubChem (CID) | 11747706 |
| ChemSpider | 9922410 |
| UNII | ZX4VV1AKUF |
| ChEMBL | CHEMBL2311179 |
| Chemical and physical data | |
| Formula | C18H24O2 |
| Molar mass | 272.39 g·mol−1 |
| 3D model (Jmol) | Interactive image |
| |
| |
Dienolone (developmental code name RU-3118; online product names Trenazone,[1] Dienazone), or nordienolone, also known as 19-nor-δ9(10)-testosterone, δ9(10)-nandrolone, or estra-4,9(10)-dien-17β-ol-3-one, is a synthetic androgenic-anabolic steroid (AAS) of the 19-nortestosterone group that was never marketed.[2] It has been found to possess slightly lower affinity for the androgen receptor (AR) and progesterone receptor (PR) relative to nandrolone in rat and rabbit tissue bioassays, whereas trenbolone was found to possess the same affinity for the AR as dienolone but several-fold increased affinity for the PR.[3] Dienedione (the 17-keto analogue of dienolone, also known as 19-nor-4,9-androstadienedione) is thought to be a prohormone of dienolone,[4] while methyldienolone and ethyldienolone are orally active 17α-alkylated AAS derivatives of dienolone.[5][6] In contrast, dienogest, the 17α-cyanomethyl derivative of dienolone, is a potent progestogen and antiandrogen.[7]
See also
- Trenbolone (trienolone)
References
- ↑ Waller CC, McLeod MD (2014). "A simple method for the small scale synthesis and solid-phase extraction purification of steroid sulfates". Steroids. 92: 74–80. doi:10.1016/j.steroids.2014.09.006. PMID 25286236.
- ↑ https://isomerdesign.com/Cdsa/HC/StatusDecisions/A-2013-00235%20-%20PDFs/C-Dienolone-2011-08-12.pdf
- ↑ Ojasoo T, Delettré J, Mornon JP, Turpin-VanDycke C, Raynaud JP (1987). "Towards the mapping of the progesterone and androgen receptors". J. Steroid Biochem. 27 (1-3): 255–69. PMID 3695484.
- ↑ https://www.deadiversion.usdoj.gov/fed_regs/rules/2009/fr1204.htm
- ↑ Detlef Thieme; Peter Hemmersbach (18 December 2009). Doping in Sports. Springer Science & Business Media. pp. 103, 157, 162–164. ISBN 978-3-540-79088-4.
- ↑ Charles D. Kochakian (6 December 2012). Anabolic-Androgenic Steroids. Springer Science & Business Media. pp. 380–381. ISBN 978-3-642-66353-6.
- ↑ Foster RH, Wilde MI (1998). "Dienogest". Drugs. 56 (5): 825–33; discussion 834–5. PMID 9829156.