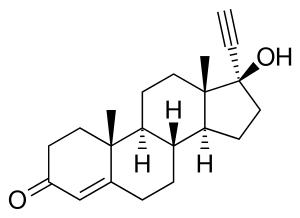
Ethisterone
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Etherone, Ethisteron, Luteosterone, Lutocyclin, Lutocylol, Pranone, Progesteron lingvalete, Progestoral, Proluton C, Syngestrotabs, Trosinone |
| ATC code | G03DC04 (WHO) |
| Identifiers | |
| |
| Synonyms | Etisteron, Pregnin, Ethindrone, Ethinyltestosterone, Ethynyltestosterone |
| CAS Number |
434-03-7 |
| PubChem (CID) | 5284557 |
| ChemSpider |
4447612 |
| UNII |
Verifiedfields = changed P201BVY1MJ Verifiedfields = changed |
| ChEBI |
CHEBI:34749 |
| ChEMBL |
CHEMBL241694 |
| Chemical and physical data | |
| Formula | C21H28O2 |
| Molar mass | 312.446 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
| | |
Ethisterone (INN, USAN, BAN) (brand names Pranone, Progestoral, Lutocylol, Proluton C, many others), also known as 17α-ethinyltestosterone, pregneninolone, or anhydrohydroxyprogesterone, is a steroidal progestin with androgenic activity which is derived from testosterone and was introduced for medical use in 1939.[1][2][3] It was the second progestogen to be marketed (intramuscular progesterone was introduced as Proluton in 1934) and was both the first orally active progestogen and the first progestin (or synthetic progestogen) to be introduced.[4][5][6] Although ethisterone has largely been superseded by newer drugs and is now little used, it continues to be available in some countries.[2] Moreover, the 19-nortestosterone progestins, such as norethisterone, are derived from ethisterone and are widely used as hormonal contraceptives and for other indications.
Pharmacology
Ethisterone is described as a relatively weak progestogen, similarly to its analogue dimethisterone.[7]
Androgenic activity
Based on in vitro research, ethisterone and norethisterone are about equipotent in their EC50 values for the androgen receptor (AR), whereas, conversely, norethisterone shows markedly increased potency relative to ethisterone in terms of its EC50 for the progesterone receptor (PR).[8] As such, there is a considerable separation in the ratios of androgenic and progestogenic activity for ethisterone and norethisterone.[8] Moreover, at the larger dosages in which it is used to achieve equivalent progestogenic effect, ethisterone has strong androgenic effects relative to norethisterone and other 19-nortestosterone progestins, and this has limited its clinical use.[9][10]
Due to its androgenic activity, ethisterone has been associated with the masculinization of female fetuses in women who have taken it during pregnancy.[11]
Estrogenic activity
High dosages of norethisterone and noretynodrel, which are 19-nortestosterone derivatives of ethisterone, were found to be associated with high rates of estrogenic side effects such as breast enlargement in women and gynecomastia in men, as well as with improvement of menopausal symptoms in postmenopausal women.[12] In contrast, ethisterone and other progestogens such as progesterone and hydroxyprogesterone caproate were not associated with such effects, suggesting that they are either not estrogenic or are only weak so.[12][13] As such, ethisterone does not appear to share the estrogenic activity of norethisterone and noretynodrel.[12][13]
Chemistry
Ethisterone is also known by the following synonyms:[1]
- 17α-Ethynyltestosterone (or simply ethinyltestosterone or ethynyltestosterone)
- Pregn-4-en-20-yn-17β-ol-3-one (or simply pregneninolone or pregnenynolone)[14][15]
- 20,21-Anhydro-17β-hydroxyprogesterone (or simply anhydrohydroxyprogesterone)[16]
- 17α-Ethynylandrost-4-en-17β-ol-3-one
Closely related analogues of ethisterone include vinyltestosterone, allyltestosterone, methyltestosterone, ethyltestosterone, and propyltestosterone.
History
Ethisterone was synthesized in 1938 by Hans Herloff Inhoffen, Willy Logemann, Walter Hohlweg, and Arthur Serini at Schering AG in Berlin.[17] It was derived from testosterone via ethynylation at the C17α position, and it was hoped, that, analogously to estradiol and ethinyl estradiol, ethisterone would be an orally active form of testosterone.[18] However, the androgenic activity of ethisterone was attenuated and it showed considerable progestogenic activity.[18] As such, it was developed as a progestogen instead and was marketed in Germany in 1939 as Proluton C and by Schering in the U.S. in 1945 as Pranone, among other brand names.[6][19]
See also
- 19-Norprogesterone
- 17α-Methylprogesterone
- List of steroidal progestogens
- List of androgens/anabolic steroids
References
- 1 2 J. Elks (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. p. 508. ISBN 978-1-4757-2085-3.
- 1 2 Index Nominum 2000: International Drug Directory. Taylor & Francis. January 2000. pp. 413–. ISBN 978-3-88763-075-1.
- ↑ Dr. Ian Morton; I.K. Morton; Judith M. Hall (31 October 1999). Concise Dictionary of Pharmacological Agents: Properties and Synonyms. Springer Science & Business Media. pp. 115–. ISBN 978-0-7514-0499-9.
- ↑ Gray Huntington Twombly (1947). Endocrinology of Neoplastic Diseases: A Symposium by Eighteen Authors. Oxford University Press. p. 7.
- ↑ William Andrew Publishing (22 October 2013). Pharmaceutical Manufacturing Encyclopedia, 3rd Edition. Elsevier. pp. 1504–1505. ISBN 978-0-8155-1856-3.
- 1 2 Christian Lauritzen; John W. W. Studd (22 June 2005). Current Management of the Menopause. CRC Press. p. 45. ISBN 978-0-203-48612-2.
Ethisterone, the first orally effective progestagen, was synthesized by Inhoffen and Hohlweg in 1938. Norethisterone, a progestogen still used worldwide, was synthesized by Djerassi in 1951. But this progestogen was not used immediately and in 1953 Colton discovered norethynodrel, used by Pincus in the first oral contraceptive. Numerous other progestogens were subsequently synthesized, e.g., lynestrenol and ethynodiol diacetate, which were, in fact, prhormones converted in vivo to norethisterone. All these progestogens were also able to induce androgenic effects when high doses were used. More potent progestogens were synthesized in the 1960s, e.g. norgestrel, norgestrienone. These progestogens were also more androgenic.
- ↑ Robert J. Kurman (17 April 2013). Blaustein's Pathology of the Female Genital Tract. Springer Science & Business Media. pp. 390–. ISBN 978-1-4757-3889-6.
- 1 2 McRobb L, Handelsman DJ, Kazlauskas R, Wilkinson S, McLeod MD, Heather AK (2008). "Structure-activity relationships of synthetic progestins in a yeast-based in vitro androgen bioassay". J. Steroid Biochem. Mol. Biol. 110 (1-2): 39–47. doi:10.1016/j.jsbmb.2007.10.008. PMID 18395441.
- ↑ P. J. Bentley (1980). Endocrine Pharmacology: Physiological Basis and Therapeutic Applications. CUP Archive. pp. 4–. ISBN 978-0-521-22673-8.
- ↑ Richard M. Eglen; Mont R. Juchau; Gillian Edwards; et al. (6 December 2012). Progress in Drug Research: Fortschritte der Arzneimittelforschung / Progrès des recherches pharmaceutiques. Birkhäuser. pp. 72–. ISBN 978-3-0348-8863-9.
- ↑ Wilkins, Lawson; Jones, Howard W.; Holman, Gerald H.; Stempfel, Robert S. (1958). "MASOULINIZATION OF THE FEMALE FETUS ASSOCIATED WITH ADMINISTRATION OF ORAL AND INTRAMUSCULAR PROGESTINS DURING GESTATION: NON-ADRENAL FEMALE PSEUDOHERMAPHRODISM*". The Journal of Clinical Endocrinology & Metabolism. 18 (6): 559–585. doi:10.1210/jcem-18-6-559. ISSN 0021-972X.
- 1 2 3 PAULSEN CA, LEACH RB, LANMAN J, GOLDSTON N, MADDOCK WO, HELLER CG (1962). "Inherent estrogenicity of norethindrone and norethynodrel: comparison with other synthetic progestins and progesterone". J. Clin. Endocrinol. Metab. 22: 1033–9. doi:10.1210/jcem-22-10-1033. PMID 13942007.
- 1 2 TROOP RC, POSSANZA GJ (1962). "Gonadal influences on the pituitary-adrenal axis". Arch. Biochem. Biophys. 98: 444–9. PMID 13922599.
Progesterone, 17 alpha-hydroxyprogesterone caproate and ethisterone administered to 14 normal male subjects failed to cause the development of gynecomastia in any of 12 subjects, suggesting that these compounds were not estrogenic, or only weakly so.
- ↑ Roche Review ... Hoffman-La Roche, and Roche-organon. 1940.
Hohlweg, Naturwiss., 1938, 26:96, added the ethinyl radical to testosterone and obtained pregneninolone. This substance has been referred to in the literature as Δ4 pregnen-in-20-on-3-ol-17; Δ4 pregnene-in, 17-ol, 3-one; ethinyl testosterone; anhydro-oxy-progesterone; anhydro-hydroxy-progesterone; and pregneninolone.
- ↑ Inhoffen, H. H.; Hohlweg, W. (1938). "Neue per os-wirksame weibliche Keimdrüsenhormon-Derivate: 17-Aethinyl-oestradiol und Pregnen-in-on-3-ol-17". Die Naturwissenschaften. 26 (6): 96–96. doi:10.1007/BF01681040. ISSN 0028-1042.
- ↑ Davis ME, Wied GL (1957). "17α-Hydroxyprogesterone acetate: An effective progestational substance on oral administration". The Journal of Clinical Endocrinology and Metabolism. 17 (10): 1237–44. doi:10.1210/jcem-17-10-1237. PMID 13475464.
- ↑ Marc A. Fritz; Leon Speroff (28 March 2012). Clinical Gynecologic Endocrinology and Infertility. Lippincott Williams & Wilkins. pp. 963–964. ISBN 978-1-4511-4847-3.
The discovery of ethinyl substitution and oral potency led (ata the end of the 1930s) to the preparation of ethisterone, an orally active derivative of testosterone. In 1951, it was demonstrated that removal of the 19-carbon from ethisterone to form norethindrone did not destroy the oral activity, and most importantly, it changed the major hormonal effect from that of an androgen to that of a progestational agent. Accordingly, the progestational derivatives of testosterone were designated as 19-nortestosterones (denoting the missing 19-carbon).
- 1 2 Kuhl H (2011). "Pharmacology of Progestogens" (PDF). J Reproduktionsmed Endokrinol. 8 (1): 157–177.
- ↑ Klaus Roth (2014). Chemische Leckerbissen. John Wiley & Sons. p. 69. ISBN 978-3-527-33739-2.
Im Prinzip hatten Hohlweg und Inhoffen die Lösung schon 1938 in der Hand, denn ihr Ethinyltestosteron (11) war eine oral wirksame gestagene Verbindung und Schering hatte daraus bereits 1939 ein Medikament (Proluton C®) entwickelt.
- Inhoffen HH, Logemann W, Hohlweg W, Serini A (May 4, 1938). "Untersuchungen in der Sexualhormon-Reihe (Investigations in the sex hormone series)" (abstract page). Ber Dtsch Chem Ges. 71 (5): 1024–32. doi:10.1002/cber.19380710520.
- Petrow V (1970). "The contraceptive progestagens". Chem Rev. 70 (6): 713–26. doi:10.1021/cr60268a004. PMID 4098492.
- Kugener, André (2004). Tabletten der Fa. Schering (Tablets of Schering AG) Proluton C tablets c. 1939
- Quinkert G (2004). "Hans Herloff Inhoffen in His Times (1906-1992)" (abstract page). Eur J Org Chem. 2004 (17): 3727–48. doi:10.1002/ejoc.200300813.
- Sneader, Walter (2005). "Hormone analogues". Drug discovery : a history. Hoboken NJ: John Wiley & Sons. pp. 188–225. ISBN 0-471-89980-1.
- Djerassi C (2006). "Chemical birth of the pill". Am J Obstet Gynecol. 194 (1): 290–8. doi:10.1016/j.ajog.2005.06.010. PMID 16389046.