Tissue plasminogen activator
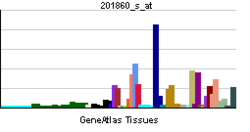
| View/Edit Human | View/Edit Mouse |
Tissue plasminogen activator (abbreviated tPA or PLAT) is a protein involved in the breakdown of blood clots. It is a serine protease (EC 3.4.21.68) found on endothelial cells, the cells that line the blood vessels. As an enzyme, it catalyzes the conversion of plasminogen to plasmin, the major enzyme responsible for clot breakdown. Because it works on the clotting system, tPA (such as alteplase, reteplase, and tenecteplase) is used in clinical medicine to treat embolic or thrombotic stroke. Use is contraindicated in hemorrhagic stroke and head trauma. The antidote for tPA in case of toxicity is aminocaproic acid.
tPA may be manufactured using recombinant biotechnology techniques. tPA created this way may be referred to as recombinant tissue plasminogen activator (rtPA). It is sold as alteplase.
Medical uses
tPA is used in some cases of diseases that feature blood clots, such as pulmonary embolism, myocardial infarction, and stroke, in a medical treatment called thrombolysis. The most common use is for ischemic stroke. It can either be administered systemically, in the case of acute myocardial infarction, acute ischemic stroke, and most cases of acute massive pulmonary embolism, or administered through an arterial catheter directly to the site of occlusion in the case of peripheral arterial thrombi and thrombi in the proximal deep veins of the leg.[3]

Ischemic stroke
There have been twelve large scale, high-quality trials of rtPA in acute ischemic stroke. A meta-analysis of these trials concluded that rtPA given within 6 hours of a stroke significantly increased the odds of being alive and independent at final follow-up, particularly in patients treated within 3 hours. However, there was an excess of mortality in treated patients in the first week after the event, mostly from intracranial haemorrhage at 7 days but mortality at final follow up was not significant between treated and untreated patients.[4]
It has been suggested that if tPA is effective in ischemic stroke, it must be administered as early as possible after the onset of stroke symptoms.[4][5] Indeed, tPA has become widely considered standard of care in acute ischemic stroke, so long as the patient presents soon after the onset of stroke symptoms.[5] Many national guidelines including the AHA have interpreted this cohort of studies as suggesting that there are specific subgroups who may benefit from tPA and thus recommend its use within a limited time window after the event. Protocol guidelines require its use intravenously within the first three hours of the event, after which its detriments may outweigh its benefits. For example, the Canadian Stroke Network guideline states "All patients with disabling acute ischemic stroke who can be treated within 4.5 hours of symptom onset should be evaluated without delay to determine their eligibility for treatment" with tPA.[6] Because of this, only about 3% of people qualify for this treatment, since most patients do not seek medical assistance quickly enough. Similarly in the United States, the window of administration used to be 3 hours from onset of symptoms, but the newer guidelines also recommend use up to 4.5 hours after symptom onset.[7] tPA appears to show benefit not only for large artery occlusions but also for lacunar strokes. Since tPA dissolves blood clots, there is risk of hemorrhage with its use.
Use of tPA in the United States in treatment of patients who are eligible for its use, no contra-indications and arrival at the treating facility less than 3 hours after onset of symptoms, is reported to have doubled from 2003 to 2011. Use on patients with mild deficits, of nonwhite race/ethnicity, and oldest old age increased. However, many patients who were eligible for treatment were not treated.[8][9]
tPA has also been given to patients with acute ischemic stroke above age 90 years old. Although a small fraction of patients 90 years and above treated with tPA for acute ischemic stroke recover, most patients have a poor 30-day functional outcome or die.[10] Nonagenarians may do as well as octogenarians following treatment with IV-tPA for acute ischemic stroke.[11] In addition, people with frostbite treated with tPA had fewer amputations than those not treated with tPA.[12]
There is consensus amongst stroke specialists that tPA is the standard of care for eligible stroke patients and benefits outweigh the risks. There is significant debate mainly in the emergency medicine community regarding recombinant tPA's effectiveness in ischemic stroke. The NNT Group on evidence-based medicine concluded that it was inappropriate to combine these twelve trials into a single analysis, because of substantial clinical heterogeneity (i.e., variations in study design, setting, and population characteristics).[13] Examining each study individually, the NNT group noted that two of these studies showed benefit to patients given tPA (and that, using analytical methods that they think flawed); four studies showed harm and had to be stopped before completion; and the remaining studies showed neither benefit nor harm. On the basis of this evidence, the NNT Group recommended against the use of tPA in acute ischaemic stroke.[13] The NNT Group notes that the case for the 3-hour time window arises largely from analysis of two trials: NINDS-2 and subgroup results from IST-3. "However, presuming that early (0-3h) administration is better than later administration (3-4.5h or 4.5-6h) the subgroup results of IST-3 suggest an implausible biological effect in which early administration is beneficial, 3-4.5h administration is harmful, and 4.5-6h administration is again beneficial."[13] Indeed, even the original publication of the IST-3 trial found that time-window effects were not significant predictors of outcome (p=0.61).[14] In the UK, concerns by stroke specialists have led to a review by the Medicines and Healthcare products Regulatory Agency.[15]
Pulmonary embolism
Pulmonary embolism (blood clots that have moved to the lung arteries) is usually treated with heparin generally followed by warfarin. If pulmonary embolism causes severe instability due to high pressure on the heart ("massive PE") and leads to low blood pressure, recombinant tPA is recommended.[16][17][18]
Recombinant tissue plasminogen activators
Recombinant tissue plasminogen activators (r-tPAs) include alteplase, reteplase, and tenecteplase (TNKase).[3]
Activase (Alteplase) is FDA-approved for treatment of myocardial infarction with ST-elevation (STEMI), acute ischemic stroke (AIS), acute massive pulmonary embolism, and central venous access devices (CVAD).[3]
Reteplase is FDA-approved for acute myocardial infarction, where it has more convenient administration and faster thrombolysis than alteplase. This is because it is a second generation engineered TPA, hence its half life is up to 20 minutes which allows it to be administered as a bolus injection rather than an infusion like Alteplase.[3]
Tenecteplase is also indicated in acute myocardial infarction, showing fewer bleeding complications but otherwise similar mortality rates after one year compared to alteplase.[3]
Additional r-tPAs, such as desmoteplase, are under clinical development.
Interactions
Tissue plasminogen activator has been shown to interact with:
Function

tPA and plasmin are the key enzymes of the fibrinolytic pathway in which tPA mediated plasmin generation occurs. To be specific, tPA cleaves the zymogen plasminogen at its Arg561 - Val562 peptide bond, into the serine protease plasmin.
Increased enzymatic activity causes hyperfibrinolysis, which manifests as excessive bleeding. Decreased activity leads to hypofibrinolysis which can result in thrombosis or embolism. In ischemic stroke patients, decreased tPA activity was reported to be associated with an increase in plasma P-selectin concentration.[24]
Tissue plasminogen activator also plays a role in cell migration and tissue remodeling.
Genetics
Tissue plasminogen activator is a protein encoded by the PLAT gene, which is located on chromosome 8. The primary transcript produced by this gene undergoes alternative splicing, producing three distinct messenger RNAs.
Development
tPA was first produced by recombinant DNA techniques at Genentech in 1982.
See also
References
- ↑ "Human PubMed Reference:".
- ↑ "Mouse PubMed Reference:".
- 1 2 3 4 5 Rivera-Bou WL, Cabanas JG, Villanueva SE (2008-11-20). "Thrombolytic Therapy". Medscape.
- 1 2 Wardlaw JM, Murray V, Berge E, del Zoppo G, Sandercock P, Lindley RL, Cohen G (June 2012). "Recombinant tissue plasminogen activator for acute ischaemic stroke: an updated systematic review and meta-analysis". Lancet. 379 (9834): 2364–72. doi:10.1016/S0140-6736(12)60738-7. PMC 3386494
 . PMID 22632907.
. PMID 22632907. - 1 2 DeMers G, Meurer WJ, Shih R, Rosenbaum S, Vilke GM (December 2012). "Tissue plasminogen activator and stroke: review of the literature for the clinician". J Emerg Med. 43 (6): 1149–54. doi:10.1016/j.jemermed.2012.05.005. PMID 22818644.
- ↑ Lindsay, Gubitz G, Bayley M, Hill MD, Davies-Schinkel C, Singh S, Phillips S (Dec 8, 2010). "Hyperacute stroke management". Canadian best practice recommendations for stroke care. Ottawa, ON: Canadian Stroke Network. Canadian Stroke Strategy Best Practices and Standards Writing Group. pp. 55–84. Retrieved 30 November 2013.
- ↑ Davis SM, Donnan GA (June 2009). "4.5 hours: the new time window for tissue plasminogen activator in stroke". Stroke. 40 (6): 2266–7. doi:10.1161/STROKEAHA.108.544171. PMID 19407232.
- ↑ Bankhead C, Agus ZS (2013-08-23). "Clot-Busting Drugs Used More Often in Stroke". Medpage Today.
- ↑ Schwamm LH, Ali SF, Reeves MJ, Smith EE, Saver JL, Messe S, Bhatt DL, Grau-Sepulveda MV, Peterson ED, Fonarow GC (August 2013). "Temporal Trends in Patient Characteristics and Treatment With Intravenous Thrombolysis Among Acute Ischemic Stroke Patients at Get With the Guidelines-Stroke Hospitals". Circ Cardiovasc Qual Outcomes. doi:10.1161/CIRCOUTCOMES.111.000095. PMID 23963655.
The frequency of IV tPA use among all AIS patients, regardless of contraindications, nearly doubled from 2003 to 2011. Treatment with tPA has expanded to include more patients with mild deficits, nonwhite race/ethnicity, and oldest old age
- ↑ Mateen FJ, Nasser M, Spencer BR, Freeman WD, Shuaib A, Demaerschalk BM, Wijdicks EF (2009). "Outcomes of intravenous tissue plasminogen activator for acute ischemic stroke in patients aged 90 years or older". Mayo Clin. Proc. 84 (4): 334–8. doi:10.1016/S0025-6196(11)60542-9. PMC 2665978
 . PMID 19339651.
. PMID 19339651. - ↑ Mateen FJ, Buchan AM, Hill MD (2010). "Outcomes of thrombolysis for acute ischemic stroke in octogenarians versus nonagenarians". Stroke. 41 (8): 1833–5. doi:10.1161/STROKEAHA.110.586438. PMID 20576948.
- ↑ Twomey JA, Peltier GL, Zera RT (2005). "An open-label study to evaluate the safety and efficacy of tissue plasminogen activator in treatment of severe frostbite". J Trauma. 59: 1350–1354. doi:10.1097/01.ta.0000195517.50778.2e.; and repeated by Bruen KJ, Ballard JR, Morris SE, Cochran A, Edelman LS, Saffle JR (June 2007). "Reduction of the incidence of amputation in frostbite injury with thrombolytic therapy". Arch Surg. 142 (6): 546–51; discussion 551–3. doi:10.1001/archsurg.142.6.546. PMID 17576891.
- 1 2 3 Newman, David (March 25, 2013). "Thrombolytics for Acute Ischemic Stroke: No benefit found". NNT Group. Retrieved 30 November 2013.
- ↑ Sandercock P, Wardlaw JM, Lindley RI, Dennis M, Cohen G, Murray G, Innes K, Venables G, Czlonkowska A, Kobayashi A, Ricci S, Murray V, Berge E, Slot KB, Hankey GJ, Correia M, Peeters A, Matz K, Lyrer P, Gubitz G, Phillips SJ, Arauz A (June 2012). "The benefits and harms of intravenous thrombolysis with recombinant tissue plasminogen activator within 6 h of acute ischaemic stroke (the third international stroke trial [IST-3]): a randomised controlled trial". Lancet. 379 (9834): 2352–63. doi:10.1016/S0140-6736(12)60768-5. PMC 3386495
 . PMID 22632908.
. PMID 22632908. - ↑ Brimelow, Adam. "Safety review into stroke clot-buster drug alteplase". BBC News. British Broadcasting Corporation. Retrieved 29 June 2015.
- ↑ Konstantinides SV, Torbicki A, Agnelli G, Danchin N, Fitzmaurice D, Galiè N, Gibbs JS, Huisman MV, Humbert M, Kucher N, Lang I, Lankeit M, Lekakis J, Maack C, Mayer E, Meneveau N, Perrier A, Pruszczyk P, Rasmussen LH, Schindler TH, Svitil P, Vonk Noordegraaf A, Zamorano JL, Zompatori M (Nov 2014). "2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism". European Heart Journal. 35 (43): 3033–69, 3069a–3069k. doi:10.1093/eurheartj/ehu283. PMID 25173341.
- ↑ National Institute for Health and Clinical Excellence. Clinical guideline 144: Venous thromboembolic diseases: the management of venous thromboembolic diseases and the role of thrombophilia testing. London, 2012.
- ↑ Hirsh J, Guyatt G, Albers GW, Harrington R, Schünemann HJ (Jun 2008). "Executive summary: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition)". Chest. 133 (6 Suppl): 71S–109S. doi:10.1378/chest.08-0693. PMID 18574259.
- ↑ Tsurupa G, Medved L (2001). "Identification and characterization of novel tPA- and plasminogen-binding sites within fibrin(ogen) alpha C-domains". Biochemistry. 40 (3): 801–8. doi:10.1021/bi001789t. PMID 11170397.
- ↑ Ichinose A, Takio K, Fujikawa K (1986). "Localization of the binding site of tissue-type plasminogen activator to fibrin". J. Clin. Invest. 78 (1): 163–9. doi:10.1172/JCI112546. PMC 329545
 . PMID 3088041.
. PMID 3088041. - ↑ Zhuo M, Holtzman DM, Li Y, Osaka H, DeMaro J, Jacquin M, Bu G (2000). "Role of tissue plasminogen activator receptor LRP in hippocampal long-term potentiation". J. Neurosci. 20 (2): 542–9. PMID 10632583.
- ↑ Orth K, Madison EL, Gething MJ, Sambrook JF, Herz J (1992). "Complexes of tissue-type plasminogen activator and its serpin inhibitor plasminogen-activator inhibitor type 1 are internalized by means of the low density lipoprotein receptor-related protein/alpha 2-macroglobulin receptor". Proc. Natl. Acad. Sci. U.S.A. 89 (16): 7422–6. doi:10.1073/pnas.89.16.7422. PMC 49722
 . PMID 1502153.
. PMID 1502153. - ↑ Parmar PK, Coates LC, Pearson JF, Hill RM, Birch NP (2002). "Neuroserpin regulates neurite outgrowth in nerve growth factor-treated PC12 cells". J. Neurochem. 82 (6): 1406–15. doi:10.1046/j.1471-4159.2002.01100.x. PMID 12354288.
- ↑ Wang J, Li J, Liu Q (August 2005). "Association between platelet activation and fibrinolysis in acute stroke patients". Neurosci Lett. 384 (3): 305–9. doi:10.1016/j.neulet.2005.04.090. PMID 15916851.
Further reading
- Rijken DC (1988). "Relationships between structure and function of tissue-type plasminogen activator". Klin. Wochenschr. 66 Suppl 12: 33–9. PMID 3126346.
- Bode W, Renatus M (1997). "Tissue-type plasminogen activator: variants and crystal/solution structures demarcate structural determinants of function". Curr. Opin. Struct. Biol. 7 (6): 865–72. doi:10.1016/S0959-440X(97)80159-5. PMID 9434908.
- Collen D, Billiau A, Edy J, De Somer P., Identification of the human plasma protein which inhibits fibrinolysis associated with malignant cells, Biochim Biophys Acta. 1977 Sep 29;499(2):194-201
- Anglés-Cano E, Rojas G (2002). "Apolipoprotein(a): structure-function relationship at the lysine-binding site and plasminogen activator cleavage site". Biol. Chem. 383 (1): 93–9. doi:10.1515/BC.2002.009. PMID 11928826.
- Wang J, Li J, Liu Q (August 2005). "Association between platelet activation and fibrinolysis in acute stroke patients". Neurosci Lett. 384 (3): 305–9. doi:10.1016/j.neulet.2005.04.090. PMID 15916851.
- Ny T, Wahlberg P, Brändström IJ (2002). "Matrix remodeling in the ovary: regulation and functional role of the plasminogen activator and matrix metalloproteinase systems". Mol. Cell. Endocrinol. 187 (1-2): 29–38. doi:10.1016/S0303-7207(01)00711-0. PMID 11988309.
- Teesalu T, Kulla A, Asser T, Koskiniemi M, Vaheri A (2002). "Tissue plasminogen activator as a key effector in neurobiology and neuropathology". Biochem. Soc. Trans. 30 (2): 183–9. doi:10.1042/BST0300183. PMID 12023848.
- Pang PT, Lu B (2004). "Regulation of late-phase LTP and long-term memory in normal and aging hippocampus: role of secreted proteins tPA and BDNF". Ageing Res. Rev. 3 (4): 407–30. doi:10.1016/j.arr.2004.07.002. PMID 15541709.
- Sheehan JJ, Tsirka SE (2005). "Fibrin-modifying serine proteases thrombin, tPA, and plasmin in ischemic stroke: a review". Glia. 50 (4): 340–50. doi:10.1002/glia.20150. PMID 15846799.
- Wang J, Rogove AD, Tsirka AE, et al. (2003). "Role of tuftsin fragment 1-3 in an animal model of intracerebral hemorrhage". Annals of Neurology. 54 (5): 655–64. doi:10.1002/ana.10750. PMID 14595655.
External links
- History of Discovery: The Tissue-Type Plasminogen Activator Story, Collen, D., Lijnen, H.R.
- Genentech Press Release 1982
- Tissue Plasminogen Activator from the American Heart Association
- Widening the Window : Strategies to buy time in treating ischemic stroke - Scientific American Magazine (August 2005)
- Study expands window for effective stroke treatment - explained on YouTube