Tularemia
| Tularemia | |
|---|---|
| Synonyms | tularaemia, Pahvant Valley plague,[1] rabbit fever,[1] deer fly fever, Ohara's fever[2] |
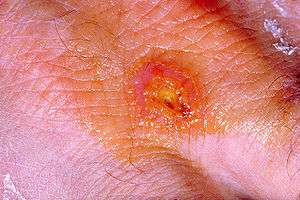 | |
| A tularemia lesion on the dorsal skin of the right hand | |
| Classification and external resources | |
| Specialty | infectious disease |
| ICD-10 | A21 |
| ICD-9-CM | 021 |
| DiseasesDB | 13454 |
| MedlinePlus | 000856 |
| eMedicine | med/2326 emerg/591 ped/2327 |
| Patient UK | Tularemia |
| MeSH | D014406 |
Tularemia is a serious infectious disease caused by the intracellular bacterium Francisella tularensis.[3] It causes fever, and sometimes ulceration at the site of entry and/or swelling of nearby lymph nodes.[4] It can cause severe pneumonia.[4]
A Gram-negative coccobacillus, Francisella tularensis has several subspecies with varying degrees of virulence. The most important of those is F. tularensis tularensis (Type A), which is found in lagomorphs (rabbits, hares and pikas) in North America, and it is highly virulent in humans and domestic rabbits. F. tularensis palaearctica (Type B) occurs mainly in aquatic rodents (beavers, muskrats) in North America and in hares and small rodents in northern Eurasia. It is less virulent for humans and rabbits.[5] The primary vectors are ticks and deer flies, but the disease can also be spread through other arthropods.[3] The disease is named after Tulare County, California.
Signs and symptoms
Depending on the site of infection, tularemia has six characteristic clinical variants: ulceroglandular (the most common type representing 75% of all forms), glandular, oropharyngeal, pneumonic, oculoglandular, and typhoidal.[6]
The incubation period for tularemia is one to 14 days; most human infections become apparent after three to five days.[7] In most susceptible mammals, the clinical signs include fever, lethargy, loss of appetite, signs of sepsis, and possibly death. Nonhuman mammals rarely develop the skin lesions seen in people. Subclinical infections are common, and animals often develop specific antibodies to the organism. Fever is moderate or very high, and tularemia bacilli can be isolated from blood cultures at this stage. The face and eyes redden and become inflamed. Inflammation spreads to the lymph nodes, which enlarge and may suppurate (mimicking bubonic plague). Lymph node involvement is accompanied by a high fever.
Death occurs in less than 1% of cases if therapy is initiated promptly. Mortality was 7% for untreated cases, and the disease responds well to antibiotics. The exact cause of death is unclear, but it is thought to be a combination of multiple organ system failures.
Cause

The bacteria can penetrate into the body through damaged skin, mucous membranes, and inhalation. Humans are most often infected by tick bite or through handling an infected animal. Ingesting infected water, soil, or food can also cause infection. Hunters are at a higher risk for this disease because of the potential of inhaling the bacteria during the skinning process. It has been contracted from inhaling particles from an infected rabbit ground up in a lawnmower (see below). Tularemia is not spread directly from person to person.[8]
Francisella tularensis is an intracellular bacterium, meaning it is able to live as a parasite within host cells. It primarily infects macrophages, a type of white blood cell, thus is able to evade the immune system. The course of disease involves the spread of the organism to multiple organ systems, including the lungs, liver, spleen, and lymphatic system. The course of disease is different depending on the route of exposure. Mortality in untreated (before the antibiotic era) patients has been as high as 50% in the pneumoniac and typhoidal forms of the disease, which however account for less than 10% of cases.[9]
Pathology
In lymph node biopsies, the typical histopathologic pattern is characterized by geographic areas of necrosis with neutrophils and necrotizing granulomas. The pattern is non specific and similar to other infectious lymphadenopathies.[10]
The laboratorial isolation of F. tularensis requires special media such as buffered charcoal and yeast extract (BCYE). It cannot be isolated in the routine culture media because of the need for sulfhydryl group donors (such as cysteine). The microbiologist must be informed when tularemia is suspected not only to include the special media for appropriate isolation, but also to ensure that safety precautions are taken to avoid contamination of laboratory personnel.
Serological tests (detection of antibodies in the serum of the patients) are available and widely used. Cross reactivity with Brucella can confuse interpretation of the results, so diagnosis should not rely only on serology. Molecular methods such as PCR are available in reference laboratories.
Prevention
An attenuated, live vaccine is available, but its use is only for high risk groups. Its use as postexposure prophylaxis is not recommended. Other preventive measures include the prompt removal of ticks, and the use of rubber gloves and eye protection when handling potentially infected wild animals.[11]
Treatment
The drug of choice for tularemia has historically been streptomycin or tetracycline-class drugs such as doxycycline.[12] Gentamicin may also be used as it is easier to obtain than streptomycin.[12] There is tentative evidence to support the use of fluoroquinolones.[12]
Epidemiology
The disease is endemic in North America and parts of Europe and Asia. The most common mode of transmission is via arthropod vectors. Ticks involved include Amblyomma, Dermacentor, Haemaphysalis, and Ixodes.[13] Rodents, rabbits, and hares often serve as reservoir hosts,[14] but waterborne infection accounts for 5 to 10% of all tularemia in the US.[15] Tularemia can also be transmitted by biting flies, particularly the deer fly Chrysops discalis. Individual flies can remain infective for 14 days and ticks for over two years. Tularemia may also be spread by direct contact with contaminated animals or material, by ingestion of poorly cooked flesh of infected animals or contaminated water, or by inhalation.
In the United States, although records show that tularemia was never particularly common, incidence rates continued to drop over the course of the 20th century, so between 1990 and 2000, the rate was less than 1 per 1,000,000, meaning the disease is extremely rare in the US today.[16]
In Sweden over a period from 1984–2012 a total of 4,830 cases of tularemia occurred (most of the infections were acquired within the country). About 1.86 cases per 100,000 persons occur each year with higher rates in those between 55 and 70.[17]
Outbreaks
From May to October 2000, an outbreak of tularemia in Martha's Vineyard resulted in one fatality, and brought the interest of the CDC as a potential investigative ground for aerosolized Francisella tularensis. For a time, Martha's Vineyard was identified as the only place in the world where documented cases of tularemia resulted from lawn mowing.[18] However, in May 2015[19] a resident of Lafayette, Colorado died from aerosolized F. tularensis which was connected to lawn mowing, highlighting a new vector of risk.
An outbreak of tularemia occurred in Kosovo in 1999-2000.[20]
In 2004, three researchers at Boston University Medical Center were accidentally infected with F. tularensis, after apparently failing to follow safety procedures.[21]
In 2005, small amounts of F. tularensis were detected in the Mall area of Washington, DC the morning after an antiwar demonstration on September 24, 2005. Biohazard sensors were triggered at six locations surrounding the Mall. While thousands of people were potentially exposed, no infections were reported. The detected bacteria likely originated from a natural source, not from a bioterror attempt.[22]
In 2005 an outbreak occurred in Germany amongst participants in a hare hunt. Approximately 27 people came into contact with contaminated blood and meat after the hunt. Ten of the exposed, aged 11 – 73, developed tularemia. One of these died due to complications caused by chronic heart disease.[23]
Tularemia is endemic in the Gori region of the Eurasian country of Georgia. The last outbreak was in 2006.[24] The disease is also endemic on the uninhabited Pakri Islands off the northern coast of Estonia. Used for bombing practice by Soviet forces, chemical and bacteriological weapons may have been dropped on these islands.[25]
In 2014, there were at least five reported cases of tularemia in Colorado.[19] In the summer of 2015, a popular hiking area just north of Boulder, Colorado was identified as a site of animal infection and signs were posted to warn hikers. The death of a Colorado resident in May 2015[19] was connected to aerosolized F. tularensis as a result of lawn mowing.
In July 2007, an outbreak was reported in the Spanish autonomous region of Castile and León and traced to the plague of voles infesting the region. Another outbreak had taken place ten years before in the same area.[26]
There are some hundreds to a thousand outbreaks of tularemia yearly in Finland, the same goes to neighboring Sweden.
In January 2011, researchers searching for brucellosis among feral hog populations in Texas discovered widespread tularemia infection or evidence of past infection in feral hog populations of at least two Texas counties,[27] even though tularemia is not normally associated with pigs at all. Precautions were recommended for those who hunt, dress, or prepare feral hogs. Since feral hogs roam over large distances, there is concern that tularemia may spread or already be present in feral hogs over a very wide geographic area.
In November 2011, it was found in Tasmania, Australia. Reports claimed it to be the first in the southern hemisphere.[28] However, the causative organism was documented to have been isolated from a foot wound in the Northern Territory, Australia in 2003.[29]
History
Ancient
F. tularensis has been identified as the cause of human outbreaks in ancient Canaan in about 1715 BC and in 1075 BC.[30] A long-lasting epidemic that plagued the eastern Mediterranean in the 14th century BC was also traced back to a focus in Canaan along the Arwad-Euphrates trading route. According to Siro I. Trevisanato, this epidemic contaminated an area stretching from Cyprus to Iraq, and from Palestine to Syria, sparing Egypt (due to a quarantine) and Anatolia (owing to effective political boundaries). Subsequently, wars are believed to have spread the same disease into central Anatolia, from whence it was deliberately introduced into western Anatolia, in acts constituting the first known record of biological warfare.[31] Finally, Aegean soldiers fighting in western Anatolia returned home to their Greek islands, further spreading the same epidemic.
Modern
The tularemia bacterium was first isolated by G.W. McCoy of the U.S. Public Health Service plague lab and reported in 1912.[32][33] Scientists determined tularemia could be dangerous to humans; a human being may catch the infection after contacting an infected animal. The ailment soon became associated with hunters, cooks and agricultural workers.[34]
Biological weapon
The Centers for Disease Control and Prevention (CDC) regard F. tularensis as a viable biological warfare agent, and it has been included in the biological warfare programs of the United States, Soviet Union and Japan at various times.[35] A former Soviet biological weapons scientist, Kanatjan Alibekov, has alleged that an outbreak of tularemia among German soldiers shortly before the siege of Stalingrad was due to the release of F. tularensis by Soviet forces. Others who have studied the pathogen "propose that an outbreak resulting from natural causes is more likely".[36][37] In the US, practical research into using rabbit fever as a biological warfare agent took place in 1954 at Pine Bluff Arsenal, Arkansas, an extension of the Camp Detrick program.[38] It was viewed as an attractive agent because:
- it is easy to aerosolize
- it is highly infective; between 10 and 50 bacteria are sufficient to infect victims
- it is nonpersistent and easy to decontaminate (unlike anthrax)
- it is highly incapacitating to infected persons
- it has comparatively low lethality, which is useful where enemy soldiers are in proximity to noncombatants, e.g. civilians
The Schu S4 strain was standardized as "Agent UL" for use in the United States M143 bursting spherical bomblet. It was a lethal biological warfare agent with an anticipated fatality rate of 40 – 60%. The rate-of-action was around three days, with a duration-of-action of one to three weeks (treated) and two to three months (untreated), with frequent relapses. UL was streptomycin resistant. The aerobiological stability of UL was a major concern, being sensitive to sunlight, and losing virulence over time after release. When the 425 strain was standardized as "agent JT" (an incapacitant rather than lethal agent), the Schu S4 strain's symbol was changed again to SR.
Both wet and dry types of F. tularensis (identified by the codes TT and ZZ) were examined during the "Red Cloud" tests, which took place from November 1966 to February 1967 in the Tanana Valley, Alaska.[39]
No vaccine is available to the general public.[40] The best way to prevent tularemia infection is to wear rubber gloves when handling or skinning wild lagomorphs and rodents, avoid ingesting uncooked wild game and untreated water sources, wear long-sleeved clothes, and use an insect repellent to prevent tick bites.[41]
References
- 1 2 Rapini, Ronald P.; Bolognia, Jean L.; Jorizzo, Joseph L. (2007). Dermatology: 2-Volume Set. St. Louis: Mosby. ISBN 1-4160-2999-0.
- ↑ James, William D.; Berger, Timothy G. (2006). Andrews' Diseases of the Skin: clinical Dermatology. Saunders Elsevier. p. 286. ISBN 0-7216-2921-0.
- 1 2 Ryan KJ, Ray CG (editors) (2004). Sherris Medical Microbiology (4th ed.). McGraw Hill. pp. 488–90. ISBN 0-8385-8529-9.
- 1 2 "Signs & Symptoms". Centers for Disease Control and Prevention. Page last reviewed: October 26, 2015
- ↑ Pearson A (1998). Zoonoses: biology, clinical practice, and public health control (Soulsby EJ, Palmer SL eds.). Oxford [Oxfordshire]: Oxford University Press. pp. 276–9. ISBN 0-19-262380-X.
- ↑ Plourde PJ, Embree J, Friesen F, Lindsay G, Williams T; Embree; Friesen; Lindsay; Williams (June 1992). "Glandular tularemia with typhoidal features in a Manitoba child". CMAJ. 146 (11): 1953–5. PMC 1490377
 . PMID 1596844.
. PMID 1596844. - ↑ Office international des épizooties. (2000). Manual of standards for diagnostic tests and vaccines: lists A and B diseases of mammals, birds and bees. Paris, France: Office international des épizooties. pp. 494–6, 1394. ISBN 92-9044-510-6.
- ↑ "WHO Guidelines on Tularemia" Published 2007
- ↑ "Tularemia: Current, comprehensive information on pathogenesis, microbiology, epidemiology, diagnosis, treatment, and prophylaxis". CIDRAP. Retrieved 2008-09-29.
- ↑ Rosado FG, Stratton CW, Mosse CA Clinicopathologic correlation of epidemiologic and histopathologic features of pediatric bacterial lymphadenitis. Arch Pathol Lab Med. 2011 Nov;135(11):1490-3. http://www.archivesofpathology.org/doi/pdf/10.5858/arpa.2010-0581-OA
- ↑ Ryan, K.J.; Ray, C.G. (2004). Sherris Medical Microbiology (4th ed.).
- 1 2 3 Hepburn, MJ; Simpson, AJ (April 2008). "Tularemia: current diagnosis and treatment options." (PDF). Expert review of anti-infective therapy. 6 (2): 231–40. doi:10.1586/14787210.6.2.231. PMID 18380605.
- ↑ George W. Beran; James H. Steele (22 October 1994). Handbook of Zoonoses: Bacterial, rickettsial, chlamydial, and mycotic. CRC Press. pp. 117–. ISBN 978-0-8493-3205-0. Retrieved 28 October 2010.
- ↑ Mörner T (December 1992). "The ecology of tularaemia". Rev. Sci. Tech. 11 (4): 1123–30. PMID 1305858.
- ↑ Jellison WL, Owen C, Bell JF, Kohls GM (1961). "Tularemia and animal populations". Wildl Dis. 17: 1–22.
- ↑ Hayes E, Marshall S, Dennis D, et al. (March 2002). "Tularemia--United States, 1990-2000". MMWR. 51 (JULIOes=181–4): 181–4. PMID 11900351.
- ↑ Desvars A, Furberg M, Hjertqvist M, Vidman L, Sjöstedt A, Rydén P; et al. (January 2015). "Epidemiology and ecology of tularemia in Sweden, 1984–2012". Emerg Infect Dis [Internet]. 21 (1): 32. doi:10.3201/eid2101.140916.
- ↑ Feldman KA, Enscore RE, Lathrop SL, et al. (November 2001). "An outbreak of primary pneumonic tularemia on Martha's Vineyard". N. Engl. J. Med. 345 (22): 1601–6. doi:10.1056/NEJMoa011374. PMID 11757506.
- 1 2 3 http://www.dailycamera.com/lafayette-news/ci_28203252/lafayette-resident-contracts-tularemia-after-mowing-lawn-dies
- ↑ Tularemia Outbreak Investigation in Kosovo: Case Control and Environmental Studies. Emerg Infect Dis. -Reintjes R, Dedushaj I, Gjini A, Jorgensen TR, Cotter B, Lieftucht A, et al. - Retrieved 3 Jan 2012
- ↑ Smith S (2005-03-29). "City tells BU to bolster safety of its medical labs". Boston Globe. Retrieved 2007-05-09.
- ↑ Dvorak P (2005-10-02). "Health Officials Vigilant for Illness After Sensors Detect Bacteria on Mall: Agent Found as Protests Drew Thousands of Visitors". Washington Post. p. C13. Retrieved 2007-05-08.
A week after six bioterrorism sensors detected the presence of a dangerous bacterium on the Mall, health officials said there are no reports that any of the thousands of people in the nation's capital Sept. 24 have tularemia, the illness that results from exposure to the bacteria.
- ↑ Epidemiologisches Bulletin (pdf) des Robert Koch-Instituts Nr. 50 16. Dezember 2005
- ↑ According to staff at Georgia's National Center for Disease Control, there was an outbreak of tularemia in the village of Zemo Rene east of Gori in December 2005 and January 2006. Twenty-six persons tested positive for the bacteria, and 45 tested positive for antibodies. There were no fatal cases. The source was deemed to be a water spring. Previous outbreaks were in Tamarasheni (2005) and Ruisi (1997 and 1998).
- ↑ Human Impact on Groundwater Management in Northern Estonia.
- ↑ Diagnóstico de un brote de tularemia en Castilla-León (Spanish)
- ↑
- ↑ Biological war disease found in Tasmania Australian Broadcasting Corporation - Retrieved 4 Nov 2011.
- ↑ Whipp MJ, Davis JM, Lum G; et al. (2003). "Characterization of a novicida-like subspecies of Francisella tularensis isolated in Australia". Journal of Medical Microbiology. 52 (Pt 9): 839–42. doi:10.1099/jmm.0.05245-0. PMID 12909664.
- ↑ Trevisanato, Siro I. (2007) “The ‘Hittite Plague’, an Epidemic of Tularemia and the First Record of Biological Warfare, Medical Hypotheses, Vol. 69, Issue 6, pp 1371-1374.
- ↑ Trevisanato (2007), Op. cit.
- ↑ A. Tärnvik1 and L. Berglund, Tularaemia. Eur Respir J 2003; 21:361-373.
- ↑ McCoy GW, Chapin CW. Bacterium tularense, the cause of a plaguelike disease of rodents. Public Health Bull 1912;53:17–23.
- ↑ http://english.pravda.ru/main/18/90/363/14923_tularemia.html
- ↑ Dennis DT, Inglesby TV, Henderson DA, et al. (June 2001). "Tularemia as a biological weapon: medical and public health management". JAMA. 285 (21): 2763–73. doi:10.1001/jama.285.21.2763. PMID 11386933.
- ↑ Croddy E, Krcalova S (October 2001). "Tularemia, Biological Warfare, and the Battle for Stalingrad (1942-1943)". Military Medicine. 166 (10).
- ↑ Sjöstedt A (June 2007). "Tularemia: history, epidemiology, pathogen physiology, and clinical manifestations". Annals of the New York Academy of Sciences. 1105: 1–29. Bibcode:2007NYASA1105....1S. doi:10.1196/annals.1409.009. PMID 17395726.
- ↑ Kanti Ghosh, Tushar, Prelas, Mark, Viswanath, Dabir: Science and Technology of Terrorism and Counterterrorism. CRC Press, 2002. Page 97. ISBN 0-8247-0870-9
- ↑ "Fact Sheet - Red Cloud", Office of the Assistant Secretary of Defense (Health Affairs), Deployment Health Support Directorate.
- ↑ "Medscape & eMedicine Log In".
- ↑ http://www.cdc.gov/tularemia/prevention/index.html