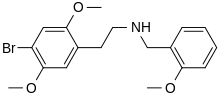
25B-NBOMe
 | |
| Legal status | |
|---|---|
| Legal status |
|
| Identifiers | |
| |
| CAS Number |
1026511-90-9 |
| PubChem (CID) | 9977044 |
| ChemSpider |
8152636 |
| UNII |
S6NAA81PHK |
| Chemical and physical data | |
| Formula | C18H22BrNO3 |
| Molar mass | 380.275 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
| | |
25B-NBOMe (NBOMe-2C-B, Cimbi-36, Nova, BOM 2-CB) is a derivative of the phenethylamine psychedelic 2C-B, discovered in 2004 by Ralf Heim at the Free University of Berlin. It acts as a potent partial agonist for the 5HT2A receptor.[1][2][3][4] Anecdotal reports from users suggest 25B-NBOMe to be an active hallucinogen at a dose of as little as 250–500 µg, making it a similar potency to other phenethylamine derived hallucinogens such as bromo-dragonfly. Duration of effects lasts about 12–16 hours.
The carbon-11 labeled version of this compound ([11C]Cimbi-36) was synthesized and validated as a radioactive tracer for positron emission tomography (PET) in Copenhagen.[5][6][7] As a 5-HT2A receptor agonist PET radioligand, [11C]Cimbi-36 was hypothesized to provide a more functional marker of these receptors. Also, [11C]Cimbi-36 is investigated as a potential marker of serotonin release and thus could serve as an indicator of serotonin levels in vivo. [11C]Cimbi-36 is now undergoing clinical trials as a PET-ligand in humans.[8][9]
Toxicity and harm potential
One case has been reported on where 25B-NBOMe was identified as the cause of death for a 17-year-old boy.[10]
25B-NBOMe has been used in clinical trials with an evaluation dose for safety consideration to humans of only 1 microgram; Such a dose is 300× lower than the dose expected to be hallucinogenic to humans and it is expected that recreational use would greatly exceed doses determined to be safe to humans.[11]
Several deaths have been attributed to its close analogue 25I-NBOMe.
Legal status
Canada
As of October 31st, 2016; 25B-NBOMe is a controlled substance (Schedule III) in Canada. http://gazette.gc.ca/rp-pr/p2/2016/2016-05-04/html/sor-dors72-eng.php
Sweden
In Sweden, the Riksdag added 25B-NBOMe to schedule I ("substances, plant materials and fungi which normally do not have medical use") as narcotics in Sweden as of August 1, 2013, published by Medical Products Agency in their regulation LVFS 2013:15 listed as 25B-NBOMe 2-(4-bromo-2,5-dimetoxifenyl)-N-(2-metoxibensyl)etanamin.[12]
United States
In November 2013, the U.S. Drug Enforcement Administration placed 25B-NBOMe (along with 25I-NBOMe and 25C-NBOMe) temporarily in Schedule I of the Controlled Substances Act, making it an illicit drug for two years.[13] In November 2015, the temporary scheduling was extended for another year.[14]
China
As of October 2015 25B-NBOMe is a controlled substance in China.[15]
Czech Republic
25B-NBOMe is banned in the Czech Republic.[16]
See also
- 2CBCB-NBOMe (NBOMe-TCB-2)
- 2CBFly-NBOMe (NBOMe-2CB-Fly)
- 25C-NBOMe (NBOMe-2C-C)
- 25I-NBOMe (NBOMe-2C-I)
- 25TFM-NBOMe (NBOMe-2C-TFM)
- 25I-NBMD (NBMD-2C-I)
- 25B-NBOH
- 25I-NBOH (NBOH-2C-I)
- 25I-NBF (NBF-2C-I)
- 5-MeO-NBpBrT
References
- ↑ Ralf Heim (February 28, 2010). "Synthese und Pharmakologie potenter 5-HT2A-Rezeptoragonisten mit N-2-Methoxybenzyl-Partialstruktur. Entwicklung eines neuen Struktur-Wirkungskonzepts." (in German). diss.fu-berlin.de. Retrieved 2013-05-10.
- ↑ Maria Silva PhD. Theoretical study of the interaction of agonists with the 5-HT2A receptor. Universität Regensburg, 2009.
- ↑ Silva ME, Heim R, Strasser A, Elz S, Dove S (January 2011). "Theoretical studies on the interaction of partial agonists with the 5-HT(2A) receptor". Journal of Computer-aided Molecular Design. 25 (1): 51–66. doi:10.1007/s10822-010-9400-2. PMID 21088982.
- ↑ Hansen, M.; Phonekeo, K.; Paine, J. S.; Leth-Petersen, S.; Begtrup, M.; Bräuner-Osborne, H.; Kristensen, J. L. (2014). "Synthesis and Structure-Activity Relationships of N-Benzyl Phenethylamines as 5-HT2A/2C Agonists". ACS Chemical Neuroscience. 5 (3): 243–9. doi:10.1021/cn400216u. PMC 3963123
 . PMID 24397362.
. PMID 24397362. - ↑ Hansen, M. (2011). Design and Synthesis of Selective Serotonin Receptor Agonists for Positron Emission Tomography Imaging of the Brain. PhD Thesis, University of Copenhagen.
- ↑ Ettrup, A.; Hansen, M.; Santini, M. A.; Paine, J.; Gillings, N.; Palner, M.; Lehel, S.; Herth, M. M.; Madsen, J.; et al. (2010). "Radiosynthesis and in vivo evaluation of a series of substituted 11C-phenethylamines as 5-HT2A agonist PET tracers". European Journal of Nuclear Medicine and Molecular Imaging. 38 (4): 681–93. doi:10.1007/s00259-010-1686-8. PMID 21174090.
- ↑ Ettrup, A.; Holm, S. R.; Hansen, M.; Wasim, M.; Santini, M. A.; Palner, M.; Madsen, J.; Svarer, C.; Kristensen, J. L.; Knudsen, G. M. (2013). "Preclinical Safety Assessment of the 5-HT2A Receptor Agonist PET Radioligand \11C]Cimbi-36". Molecular Imaging and Biology. 15 (4): 376–383. doi:10.1007/s11307-012-0609-4. PMID 23306971.
- ↑ "From molecule to man: The full CIMBI-36 story" (PDF). http://www.cimbi.dk. Retrieved 2014-01-10. External link in
|publisher=(help) - ↑ "Imanova announces the launch of a new imaging biomarker to investigate the serotonin system in psychiatric illness" (html). http://www.imanova.co.uk. Retrieved 2015-04-09. External link in
|publisher=(help) - ↑ Roxas, Gabriel (February 19, 2015). "Designer Drug Identified As Cause Of Plano Teen's Death". CBS 11 News. Retrieved 2015-02-22.
- ↑ Preclinical Safety Assessment of the 5-HT2A Receptor Agonist PET Radioligand [11CCimbi-36]
- ↑ "Föreskrifter om ändring i Läkemedelsverkets föreskrifter (LVFS 2011:10) om förteckningar över narkotika;" (PDF) (in Swedish). http://www.lakemedelsverket.se. Retrieved 2013-10-04. External link in
|publisher=(help) - ↑ http://www.justice.gov/dea/divisions/hq/2013/hq111513.shtml
- ↑ Drug Enforcement Administration (2015). "Schedules of Controlled Substances: Extension of Temporary Placement of Three Synthetic Phenethylamines in Schedule I. Final order". Fed Regist. 80 (219): 70657–9. PMID 26567439.
- ↑ "关于印发《非药用类麻醉药品和精神药品列管办法》的通知" (in Chinese). China Food and Drug Administration. 27 September 2015. Retrieved 1 October 2015.
- ↑ "Látky, o které byl doplněn seznam č. 4 psychotropních látek (příloha č. 4 k nařízení vlády č. 463/2013 Sb.)" (PDF) (in Czech). Ministerstvo zdravotnictví.