Nabilone
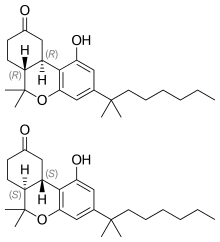 | |
 Top: (R,R)-(−)-nabilone, Center: (S,S)-(+)-nabilone, Bottom: Space-filling model of (R,R)-(−)-nabilone | |
| Clinical data | |
|---|---|
| Trade names | Cesamet, Canemes |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a607048 |
| Pregnancy category |
|
| Routes of administration | By mouth (capsules) |
| ATC code | A04AD11 (WHO) |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 20% after first-pass by the liver |
| Protein binding | similar to THC (±97%) |
| Biological half-life | 2 hours, with metabolites around 35 hours |
| Identifiers | |
| |
| CAS Number |
51022-71-0 |
| PubChem (CID) | 5284592 |
| DrugBank |
DB00486 |
| ChemSpider |
4447641 |
| UNII |
2N4O9L084N |
| KEGG |
D05099 |
| ChEMBL |
CHEMBL947 |
| ECHA InfoCard | 100.164.824 |
| Chemical and physical data | |
| Formula | C24H36O3 |
| Molar mass | 372.541 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
| (verify) | |
Nabilone is a synthetic cannabinoid with therapeutic use as an antiemetic and as an adjunct analgesic for neuropathic pain. It mimics tetrahydrocannabinol (THC), the primary psychoactive compound found naturally occurring in Cannabis.[1]
Although it does not have any indication officially (except in Mexico), nabilone is widely used as an adjunct therapy for chronic pain management. Numerous trials and case studies have demonstrated modest effectiveness for relieving fibromyalgia[2] and multiple sclerosis.[3]
Nabilone is a racemic mixture consisting of (S,S)-(+)- and (R,R)-(−)-isomers (cis-trans isomerism).
Medical uses
Nabilone is used to treat nausea and vomiting in people under chemotherapy.[1][4]
Nabilone has shown modest effectiveness in relieving fibromyalgia.[2] A 2011 systematic review of cannabinoids for chronic pain determined there was evidence of safety and modest efficacy for some conditions.[5]
The main settings that have seen published clinical trials of nabilone include movement disorders such as parkinsonism, chronic pain, dystonia and spasticity neurological disorders, multiple sclerosis, and the nausea of cancer chemotherapy. Nabilone is also effective in the treatment of inflammatory bowel disease, especially ulcerative colitis. Medical cannabis patients report that nabilone is more similar in effect to cannabidiol (CBD) than tetrahydrocannabinol (THC), indicating that it has more of a therapeutic effect on the body than a "high" effect on the mind.
A study comparing nabilone with metoclopramide, conducted before the development of modern 5-HT3 antagonist anti-emetics such as ondansetron, revealed that patients taking cisplatin chemotherapy preferred metoclopramide, while patients taking carboplatin preferred nabilone to control nausea and vomiting.[6]
Research
Nabilone is sometimes used for nightmares in PTSD, but there have not been studies longer than nine weeks, so effects of longer term use are not known.[7] Nabilone has also been used for medication overuse headache.[8]
Pharmacokinetics
Nabilone is given in 1 or 2 mg doses multiple times a day up to a total of 6 mg. It is completely absorbed from oral administration and highly plasma protein bound. Multiple P450 enzymes extensively metabolize nabilone to various metabolites that have not been fully characterized.[1]
Adverse effects
Nabilone can increase—rather than decrease—post-operative pain. In the treatment of fibromyalgia, adverse effects limits the useful dose.[2] Adverse effects of nabilone include, but are not limited to: dizziness/vertigo, euphoria, drowsiness, dry mouth, ataxia, sleep disturbance, dysphoria, headache, nausea, disorientation, depersonalization, asthenia.[1]
Society and culture
Nabilone was originally developed by Eli Lilly and Company; Lilly received FDA approval in 1985 to market it, but withdrew that approval in 1989 for commercial reasons.[9] Valeant Pharmaceuticals acquired the rights from Lilly in 2004.[9] Valeant tried and failed to get the drug approved in 2005[10] and then succeeded in 2006.[9]
In 2007 Valeant acquired the UK and EU rights to market nabilone from Cambridge Laboratories.[11]
Nabilone was approved in Austria to treat chemotherapy-induced nausea in 2013; it was already approved in Spain for the same indication and was legal in Belgium to treat glaucoma, spasticity in Multiple Sclerosis, wasting from AIDS, and chronic pain.[12]
See also
- Dronabinol
- Sativex — a prescription-only medication, sold in various countries worldwide, which contains both tetrahydrocannabinol (THC) and cannabidiol (CBD)
References
- 1 2 3 4 "Nabilone label" (PDF). FDA. May 2006.
- 1 2 3 Fine PG, Rosenfeld MJ (2013). "The endocannabinoid system, cannabinoids, and pain". Rambam Maimonides Med J (Review). 4 (4): e0022. doi:10.5041/RMMJ.10129. PMC 3820295
 . PMID 24228165.
. PMID 24228165. - ↑ Wissel J, et al. (2006). "Low dose treatment with the synthetic cannabinoid Nabilone significantly reduces spasticity-related pain : a double-blind placebo-controlled cross-over trial". J Neurol. (Research article). 253 (10): 1337–41. doi:10.1007/s00415-006-0218-8. PMID 16988792.
- ↑ "Nabilone Summary of Product Characteristics (SPC) - (eMC)". UK Electronic Medicines Compendium. August 2014.
- ↑ "Cannabinoids for treatment of chronic non-cancer pain; a systematic review of randomized trials". Br J Clin Pharmacol. 72: 735–44. Nov 2011. doi:10.1111/j.1365-2125.2011.03970.x. PMC 3243008
 . PMID 21426373.
. PMID 21426373. - ↑ Cunningham D, et al. (1988). "A randomized trial of oral nabilone and prochlorperazine compared to intravenous metoclopramide and dexamethasone in the treatment of nausea and vomiting induced by chemotherapy regimens containing cisplatin or cisplatin analogues". Eur J Cancer Clin Oncol (Randomized controlled trial). 24 (4): 685–9. doi:10.1016/0277-5379(88)90300-8. PMID 2838294.
- ↑ Canadian Agency for Drugs and Technologies in Health (Oct 2015). "Long-term Nabilone Use: A Review of the Clinical Effectiveness and Safety". CADTH Rapid Response Reports. PMID 26561692. Retrieved 19 November 2015.
- ↑ Pini, Luigi Alberto; Guerzoni, Simona; Cainazzo, Maria Michela; Ferrari, Anna; Sarchielli, Paola; Tiraferri, Ilaria; Ciccarese, Michela; Zappaterra, Maurizio (2012-11-01). "Nabilone for the treatment of medication overuse headache: results of a preliminary double-blind, active-controlled, randomized trial". The Journal of Headache and Pain. 13 (8): 677–684. doi:10.1007/s10194-012-0490-1. ISSN 1129-2377. PMC 3484259
 . PMID 23070400.
. PMID 23070400. - 1 2 3 "Valeant returns synthetic cannabinoid to USA". Pharma Times. 17 May 2006.
- ↑ "FDA turns down Valeant's anti-nausea drug". Pharma Times. 3 January 2006.
- ↑ "Cambridge Labs divests nabilone to Valeant - Pharmaceutical industry n". The Pharma Letter. February 27, 2007.
- ↑ "Cannabis Laws & Scheduling in Europe - MedicalMarijuana.eu". MedicalMarijuana.eu. Retrieved 17 November 2016.