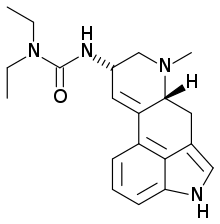
Lisuride
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | Oral, subcutaneous, transdermal |
| ATC code | G02CB02 (WHO) N02CA07 (WHO) |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 10-20% for lisuride hydrogen maleate |
| Protein binding | about 15% |
| Metabolism | Hepatic |
| Biological half-life | 2 hours |
| Excretion | renal and biliary in equal amounts |
| Identifiers | |
| |
| CAS Number |
18016-80-3 |
| PubChem (CID) | 28864 |
| IUPHAR/BPS | 227 |
| DrugBank |
DB00589 |
| ChemSpider |
26847 |
| UNII |
E0QN3D755O |
| KEGG |
D08132 |
| ChEBI |
CHEBI:51164 |
| ChEMBL |
CHEMBL157138 |
| ECHA InfoCard | 100.038.099 |
| Chemical and physical data | |
| Formula | C20H26N4O |
| Molar mass | 338.447 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
| (verify) | |
Lisuride (Dopergin, Proclacam, Revanil) is an antiparkinson agent of the iso-ergoline class, chemically related to the dopaminergic ergoline Parkinson's drugs. Lisuride is described as free base (see table on the right) and as hydrogen maleate salt.
Lisuride is used to lower prolactin and, in low doses, to prevent migraine attacks. The use of lisuride as initial anti-Parkinsonian treatment has been advocated, delaying the need for levodopa until lisuride becomes insufficient for controlling the parkinsonian disability. Preliminary trials suggest the dermal application of lisuride may be useful in the treatment of Parkinson's disease. As lisuride is very poorly absorbed when take orally and has a short half-life, continuous transdermal administration offers significant advantages and could make the compound a far more consistent therapeutic. Lisuride is not currently available in the US, as the drug was not a commercial success in comparison with other dopamine receptor agonist antiparkinsonian compounds. It is still used clinically in a number of countries in the EU and is still commercially available in the UK and China.
Mode of action
Lisuride is a dopamine and a partial agonist for several serotonin receptors. It is an antagonist at the serotonin 5-HT2B receptor.[1] It has a high affinity for the dopamine D2, D3 and D4 receptors, as well as serotonin 5-HT1A[2] and 5-HT2A/C receptors.[3] While lisuride has a similar receptor binding profile to the more well-known and chemically similar ergoloid N,N-diethyl-lysergamide (LSD) and inhibits dorsal raphe serotonergic neurons in a similar fashion to LSD, a trait which indicates both drugs in the treatment of Parkinson's disease,[4] it lacks the psychedelic effects of its sister compound. Newer findings suggest the lack of psychedelic action arises from the phenomenon of biased agonism. Stimulation of the 5-HT2A protomer within the 5-HT2A-mGlu2 receptor complex evokes psychedelic effects, while these effects do not occur during sole stimulation of monomeric 5-HT2A receptors. Accordingly, different G-proteins are involved.[5][6] Lisurid behaves as an agonist at the 5-HT2AR monomer. Since it competitively antagonises the effects of LSD, it may be regarded as a protomer antagonist of the 5-HT2A-mGluR heteromer.[7] GPCR oligomers are discrete entities and usually possess properties distinct from their parent monomeric receptors.
References
- ↑ Hofmann C, Penner U, Dorow R, Pertz HH, Jähnichen S, Horowski R, Latté KP, Palla D, Schurad B (2006). "Lisuride, a dopamine receptor agonist with 5-HT2B receptor antagonist properties: absence of cardiac valvulopathy adverse drug reaction reports supports the concept of a crucial role for 5-HT2B receptor agonism in cardiac valvular fibrosis". Clin Neuropharmacol. 29 (2): 80–6. doi:10.1097/00002826-200603000-00005. PMID 16614540.
- ↑ Marona-Lewicka D, Kurrasch-Orbaugh DM, Selken JR, Cumbay MG, Lisnicchia JG, Nichols DE (October 2002). "Re-evaluation of lisuride pharmacology: 5-hydroxytryptamine1A receptor-mediated behavioral effects overlap its other properties in rats". Psychopharmacology (Berl.). 164 (1): 93–107. doi:10.1007/s00213-002-1141-z. PMID 12373423.
- ↑ Egan CT, Herrick-Davis K, Miller K, Glennon RA, Teitler M (April 1998). "Agonist activity of LSD and lisuride at cloned 5HT2A and 5HT2C receptors". Psychopharmacology (Berl.). 136 (4): 409–14. doi:10.1007/s002130050585. PMID 9600588.
- ↑ Rogawski MA, Aghajanian GK (1979). "Response of central monoaminergic neurons to lisuride: comparison with LSD". Life Sci. 24 (14): 1289–1297. doi:10.1016/0024-3205(79)90148-6. PMID 470543.
- ↑ Moreno JL, Holloway T, Albizu L, Sealfon SC, González-Maeso J (2011). "Metabotropic glutamate mGlu2 receptor is necessary for the pharmacological and behavioral effects induced by hallucinogenic 5-HT2A receptor agonists". Neurosci. Lett. 493 (3): 76–9. doi:10.1016/j.neulet.2011.01.046. PMC 3064746
 . PMID 21276828.
. PMID 21276828. - ↑ González-Maeso J, Ang RL, Yuen T, et al. (2008). "Identification of a serotonin/glutamate receptor complex implicated in psychosis". Nature. 452 (7183): 93–7. doi:10.1038/nature06612. PMC 2743172
 . PMID 18297054.
. PMID 18297054. - ↑ González-Maeso J et al. (2007): "Hallucinogens recruit specific cortical 5-HT(2A) receptor-mediated signaling pathways to affect behavior", Neuron, Bd. 53, S. 439. PMID 17270739