Almotriptan
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Axert |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a603028 |
| Pregnancy category |
|
| Routes of administration | Oral |
| ATC code | N02CC05 (WHO) |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 70% |
| Protein binding | 35% |
| Metabolism | Hepatic |
| Biological half-life | 3–4 hours |
| Identifiers | |
| |
| CAS Number |
154323-57-6 |
| PubChem (CID) | 123606 |
| IUPHAR/BPS | 7110 |
| DrugBank |
DB00918 |
| ChemSpider |
110198 |
| UNII |
1O4XL5SN61 |
| KEGG |
D02824 |
| ChEBI |
CHEBI:520985 |
| ChEMBL |
CHEMBL1505 |
| Chemical and physical data | |
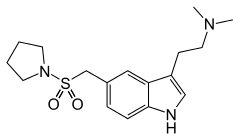
| Formula | C17H25N3O2S |
| Molar mass | 335.465 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
| | |
Almotriptan (trade name Axert and others) is a triptan drug discovered and developed by Almirall for the treatment of heavy migraine headache.
Medical uses
Almotriptan is prescribed to treat the acute headache phase of migraine attacks with or without aura.[1] Almotriptan is the only oral triptan approved in the USA for the treatment of migraine in adolescent from 12 to 17 years of age.
Efficacy
The efficacy and tolerability of almotriptan has been studied in numerous randomised, control-led trials totaling more than 4800 adults with either moderate or severe attacks of migraine. Its efficacy is significantly more effective than placebo and alleviates nausea, photophobia and phonophobia linked to migraine attacks. Almotriptan has similar efficacy as a standard dose of sumatriptan, another triptan drug, and fewer adverse effects.[2]
Contraindications
As with other triptans, almotriptan should not be used in patients with a history, symptoms or signs of ischaemic heart disease (myocardial infarction, angina pectoris, documented silent ischaemia, Prinzmetal’s angina) or severe hypertension and uncontrolled mild or moderate hypertension. Other contraindications are previous cerebrovascular accident (CVA) or transient ischaemic attack (TIA), peripheral vascular disease, severe hepatic impairment, concomitant administration of ergotamine, ergotamine derivatives (including methysergide) and other 5-HT1B/D agonists.
Side effects
Almotriptan has proved to have an adverse effects profile similar to placebo when used following the Summary of Product Characteristics instructions (see references).
Pharmacology
Mechanism of action
Like all triptans, almotriptan has a high and specific affinity for serotonin 5-HT1B/1D receptors. Binding of the drug to the receptor leads to vasoconstriction of the cranial (brain) blood vessels and thus affects the redistribution of blood flow. Almotriptan significantly increases cerebral blood flow and reduces blood flow through extracerebral cranial vessels. Even though it affects cranial blood vessels a single standard dose of almotriptan has no clinically significant effect on blood pressure or heart rate in both young and elderly healthy volunteers. Larger doses seem to slightly increase blood pressure but not beyond clinical relevance.[2]
Pharmacokinetics
Almotriptan has linear pharmacokinetics up to the 16-fold standard dose. Its biological half-life is 3 hours, and its bioavailability 70%. Cmax is observed 1.5–4 hours after oral administration, and approximately 50% of the drug is excreted unchanged in the urine. Metabolism is mediated through the enzymes MAO-A and CYP3A4 and CYP2D6 oxidation. Almotriptan clearance is moderately reduced in the elderly but does not require dose adjustment. Sex does not alter the pharmacokinetics of the drug. People with moderate-to-severe renal dysfunction are recommended to use only half the dose.[3]
Interactions
As mentioned previously, almotriptan is metabolized mainly by MAO-A and to lesser extent by CYP3A4 and CYP2D6. Studies of drugs used as preventive against migraine (propranolol and verapamil), anti-depressants (moclobemide and fluoxetine) yielded results that showed significant altering of the pharmacokinetics of almotriptan though they were deemed not clinically relevant.[2]
Society and culture
Brand names
Brand names include Axert (US, Canada), Almogran (Belgium, Denmark, Finland, France, Germany, Italy, Ireland Portugal, Spain, the United Kingdom, the Netherlands, Sweden, Switzerland, South Korea…), Almotrex (Italy) and Amignul (Spain).
References
- ↑ "Almotriptan Facts and Comparisons". Drugs.com. Retrieved 7 October 2012.
- 1 2 3 Keam, SJ; Goa, KL; Figgitt, DP (2002). "Almotriptan: a review of its use in migraine.". Drugs. 62 (2): 387–414. doi:10.2165/00003495-200262020-00010. PMID 11817980.
- ↑ McEnroe, JD; Fleishaker, JC (2005). "Clinical pharmacokinetics of almotriptan, a serotonin 5-HT(1B/1D) receptor agonist for the treatment of migraine.". Clinical pharmacokinetics. 44 (3): 237–46. doi:10.2165/00003088-200544030-00002. PMID 15762767.
- Early vs. non-early intervention in acute migraine-'Act when Mild (AwM)'. A double-blind, placebo-controlled trial of almotriptan. Goadsby PJ et al., Cephalalgia. 2008 Apr;28(4):383-91
- Triptans (serotonin, 5-HT1B/1D agonists) in migraine: detailed results and methods of a meta-analysis of 53 trials. MD Ferrari et al., (Cephalalgia 2002; 22:633-658)
- Almotriptan is effective and well tolerated in migraine patients who respond poorly to oral sumatriptan: A double-blind, randomized trial, Diener HC (Headache. 2005 Jul-Aug;45(7):874-82)
- Triptans and CNS side effects: pharmacokinetic and metabolic mechanisms. DW Dodick et al. Cephalalgia. 2004 Jun;24(6):417-24
- Triptans and chest symptoms: the role of pulmonary vasoconstriction. DW Dodick (Cephalalgia, 2004,24, 298–304)
- Almotriptan improves response rates when treatment is within 1 hour of migraine onset. AJ Dowson et al., (Headache 2004; 44: 318-322)
- A long-term open-label study of oral almotriptan 12.5 mg for the treatment of acute migraine. NT. Mathew; for the Oral Almotriptan Study Group (Headache. 2002;42:32-40)
- Almotriptan is an effective and well-tolerated treatment for migraine pain: results of a randomized, double-blind, placebo-controlled clinical trial. AJ Dowson et al. (Cephalalgia 2002; 22, 453-461)
- Tolerability and efficacy of almotriptan in the long-term treatment of migraine, J. Pascual et al. (Eur Neurol 2001;45:206-213)
- Consistent efficacy and tolerability of almotriptan in the acute treatment of multiple migraine attacks: results of a large, randomized, double-blind, placebo-controlled study. J. Pascual et al. Cephalalgia. 2000;20:588-596
- Almotriptan in the treatment of migraine attacks in clinical practice: results of the TEA 2000 observational study, Pascual J et al., Neurologia. 2003 Jan-Feb;18(1):7-17.
- Early intervention with almotriptan: results of the AEGIS trial (AXERT Early Migraine Intervention Study). Mathew et al., Headache. 2007 Feb;47(2):189-98
- Evaluation of efficacy, tolerability, and treatment satisfaction with almotriptan in 3 consecutive migraine attacks. The migraine—satisfaction with treatment: reality with Almogran study. Massiouu H et al., Eur Neurol. 2006;55(4):198-203
- Triptans in the treatment of migraine: drug selection by means of the SOJA method Expert Opin Pharmacother. Janknegt J,2007 Oct;8 Suppl 1:S15-30
- Patient preference in migraine therapy. A randomized, open-label, crossover clinical trial of acute treatment of migraine with oral almotriptan and rizatriptan Díez FI et al., J Neurol. 2007 Feb;254(2):242-9
- Use of the sustained pain-free plus no adverse events endpoint in clinical trials of triptans in acute migraine. Dodick DW et al. CNS Drugs. 2007;21(1):73-82
- A pharmacoeconomic evaluation of oral triptans in the treatment of migraine in Italy. Gori S. et al. Minerva Med. 2006 Dec;97(6):467-77
- Management costs of chest and CNS-related adverse events associated with the treatment of acute migraine attacks with oral triptans. Slof J et al., Neurologia. 2005 Jul-Aug;20(6):290-8
- Efficacy, speed of action and tolerability of almotriptan in the acute treatment of migraine: pooled individual patient data from four randomized, double-blind, placebo-controlled clinical trials. Dahlöf CG et al. Cephalalgia 2006;26:400–408
- Characteristics of migraine attacks and responses to almotriptan treatment: a comparison of menstrually related and nonmenstrually related migraines. Diamond ML et al., Headache 2008;48:248–258
External links
- R&D page by Almirall Prodesfarma
- Axert Official homepage by Ortho-McNeil
- Axert prescribing information