Cannabidiol
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Epidiolex |
| AHFS/Drugs.com | International Drug Names |
| ATC code | None |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 13–19% (oral),[2] 11–45% (mean 31%; inhaled)[3] |
| Biological half-life | 9 h[2] |
| Identifiers | |
| |
| CAS Number |
13956-29-1 |
| PubChem (CID) | 644019 |
| IUPHAR/BPS | 4150 |
| ChemSpider |
24593618 |
| UNII |
19GBJ60SN5 |
| ChEBI |
CHEBI:69478 |
| Chemical and physical data | |
| Formula | C21H30O2 |
| Molar mass | 314.4636 |
| 3D model (Jmol) | Interactive image |
| Melting point | 66 °C (151 °F) |
| Boiling point |
180 °C (356 °F) (range: 160–180 °C)[4] |
| |
| |
| | |
Cannabidiol (CBD) (INN)[5] is one of at least 113 active cannabinoids identified in cannabis.[6][7] It is a major phytocannabinoid, accounting for up to 40% of the plant's extract.[8] CBD is considered to have a wide scope of potential medical applications – due to clinical reports showing the lack of side effects, particularly a lack of psychoactivity (as is typically associated with ∆9-THC), and non-interference with several psychomotor learning and psychological functions.
Research

Epilepsy
Dravet syndrome is a rare form of epilepsy that is difficult to treat. It is a catastrophic form of intractable epilepsy that begins in infancy. Initial seizures are most often prolonged events and in the second year of life other seizure types begin to emerge.[9] A number of high profile and anecdotal reports have sparked interest in treatment of Dravet syndrome with cannabidiol.[10]
Some cannabis/hemp extract preparations containing CBD are marketed as dietary supplements and claim efficacy against Dravet Syndrome. One such preparation is marketed under the tradename Charlotte's Web Hemp Extract.[11][12] Blended/suspended in oil, the supplement contains 0.3% THC (see Legal status below, classified as hemp).
GW Pharmaceuticals is seeking FDA approval to market a liquid formulation of pure plant-derived CBD, under the trade name Epidiolex (containing 99% cannabidiol and less than 0.10% Δ9-THC) as a treatment for Dravet syndrome. Epidiolex was granted fast-track status and is in late stage trials following positive early results from the drug.[10][13][14][15][16]
A 2014 review stated that cannabidiol has been claimed, anecdotally, to be of benefit in helping people with epilepsy. Information in the review stated that there is no established mechanism of action and the lack of high-quality evidence in this area precluded conclusions being drawn.[17]
A 2016 review states that because of the poor quality of available data, "no conclusions can be drawn" about the effectiveness of cannabidiol as an epilepsy treatment.[18]
Psychosis
There is tentative evidence that CBD had an anti-psychotic effect, but research in this area is limited.[19][20]
Safety
CBD safety in humans has been studied in multiple small studies, suggesting that it is well tolerated at doses of up to 1500 mg/day (p.o.) or 30 mg (i.v.).[21]
Pharmacodynamics
Cannabidiol has a very low affinity for CB1 and CB2 receptors but acts as an indirect antagonist of their agonists.[22][23] While one would assume that this would cause cannabidiol to reduce the effects of THC, it may potentiate THC's effects by increasing CB1 receptor density or through another CB1-related mechanism.[24] It may also extend the duration of the effects of THC via inhibition of the cytochrome P-450-3A and 2C enzymes.[25]
Cannabidiol has been found to be an antagonist at the putative cannabinoid receptor, GPR55, a GPCR expressed in the caudate nucleus and putamen.[26] Cannabidiol has also been shown to act as a 5-HT1A receptor partial agonist,[27] an action which may be involved in its antidepressant,[28][29] anxiolytic,[29][30] and neuroprotective[31][32] effects. Cannabidiol is an allosteric modulator of μ and δ-opioid receptors.[33] Cannabidiol's pharmacological effects have also been attributed to PPAR-γ receptor agonism and intracellular calcium release.[8]
Research suggests that CBD may exert some of its pharmacological action through its inhibition of FAAH, which may in turn increase the levels of endocannabinoids, such as anandamide, produced by the body.[8] It has also been speculated that some of the metabolites of CBD have pharmacological effects that contribute to the biological activity of CBD.[34]
Pharmacokinetic interactions
There is some preclinical evidence to suggest that cannabidiol may reduce THC clearance, modestly increasing THC's plasma concentrations resulting in a greater amount of THC available to receptors, increasing the effect of THC in a dose-dependent manner.[35][36] Despite this, the available evidence in humans suggests no significant effect of CBD on THC plasma levels.[37]
Pharmaceutical preparations
Nabiximols (USAN, trade name Sativex) is an aerosolized mist for oral administration containing a near 1:1 ratio of CBD and THC. The drug was approved by Canadian authorities in 2005 to alleviate pain associated with multiple sclerosis.[38][39] Epidiolex, a drug with cannabidiol as its active pharmaceutical ingredient, received orphan drug status in the United States for treatment of Dravet syndrome in July 2015.[40]
Epidiolex is an oil formulation of CBD extracted from the cannabis plant undergoing clinical trials for refractory epilepsy syndromes.[41]
Chemistry
Cannabidiol is insoluble in water but soluble in organic solvents such as pentane. At room temperature, it is a colorless crystalline solid.[42] In strongly basic media and the presence of air, it is oxidized to a quinone.[43] Under acidic conditions it cyclizes to THC.[44] The synthesis of cannabidiol has been accomplished by several research groups.[45][46][47]
Biosynthesis
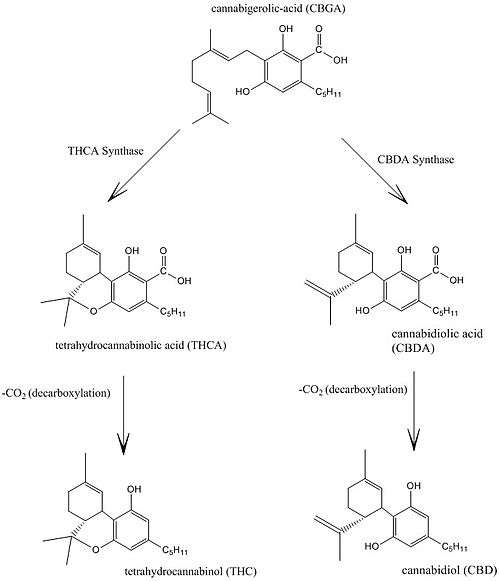
Cannabis produces CBD-carboxylic acid through the same metabolic pathway as THC, until the last step, where CBDA synthase performs catalysis instead of THCA synthase.[48]
Isomerism

| 7 double bond isomers and their 30 stereoisomers | ||||||||
|---|---|---|---|---|---|---|---|---|
| Formal numbering | Terpenoid numbering | Number of stereoisomers | Natural occurrence | Convention on Psychotropic Substances Schedule | Structure | |||
| Short name | Chiral centers | Full name | Short name | Chiral centers | ||||
| Δ5-cannabidiol | 1 and 3 | 2-(6-isopropenyl-3-methyl-5-cyclohexen-1-yl)-5-pentyl-1,3-benzenediol | Δ4-cannabidiol | 1 and 3 | 4 | No | unscheduled | -5-pentyl-1%2C3-benzenediol.png) |
| Δ4-cannabidiol | 1, 3 and 6 | 2-(6-isopropenyl-3-methyl-4-cyclohexen-1-yl)-5-pentyl-1,3-benzenediol | Δ5-cannabidiol | 1, 3 and 4 | 8 | No | unscheduled | -5-pentyl-1%2C3-benzenediol.png) |
| Δ3-cannabidiol | 1 and 6 | 2-(6-isopropenyl-3-methyl-3-cyclohexen-1-yl)-5-pentyl-1,3-benzenediol | Δ6-cannabidiol | 3 and 4 | 4 | ? | unscheduled | -5-pentyl-1%2C3-benzenediol.png) |
| Δ3,7-cannabidiol | 1 and 6 | 2-(6-isopropenyl-3-methylenecyclohex-1-yl)-5-pentyl-1,3-benzenediol | Δ1,7-cannabidiol | 3 and 4 | 4 | No | unscheduled | -5-pentyl-1%2C3-benzenediol.png) |
| Δ2-cannabidiol | 1 and 6 | 2-(6-isopropenyl-3-methyl-2-cyclohexen-1-yl)-5-pentyl-1,3-benzenediol | Δ1-cannabidiol | 3 and 4 | 4 | Yes | unscheduled | -5-pentyl-1%2C3-benzenediol.png) |
| Δ1-cannabidiol | 3 and 6 | 2-(6-isopropenyl-3-methyl-1-cyclohexen-1-yl)-5-pentyl-1,3-benzenediol | Δ2-cannabidiol | 1 and 4 | 4 | No | unscheduled | -5-pentyl-1%2C3-benzenediol.png) |
| Δ6-cannabidiol | 3 | 2-(6-isopropenyl-3-methyl-6-cyclohexen-1-yl)-5-pentyl-1,3-benzenediol | Δ3-cannabidiol | 1 | 2 | No | unscheduled | -5-pentyl-1%2C3-benzenediol.png) |
See also: Tetrahydrocannabinol#Isomerism, Abnormal cannabidiol.
Society and culture
Natural sources
Selective breeding by growers in the USA dramatically lowered the CBD content of cannabis; their customers preferred varietals that were more mind-altering due to a higher THC, lower CBD content.[50] To meet the demands of medical cannabis patients, growers are currently developing more CBD-dominant strains.[51]
Legal status
Cannabidiol is not scheduled by the Convention on Psychotropic Substances. CBD does not cause the "high" associated with the ∆9-THC in marijuana. As the legal landscape and understanding about the differences in medical cannabinoids unfolds, it will be increasingly important to distinguish “medical marijuana” (with noted varying degrees of psychotropic effects and deficits in executive function) – from “medical CBD” (in which the high CBD and low THC content may mitigate psychosis).[52][53][54]
Various breeds/strains of "medical marijuana" are found to have a significant variety in the ratios of CBD-to-THC and are known to contain other non-psychotropic cannabinoids.[55][56] However it is only the amount of ∆9-THC that chemically gives a legal determination as to whether the plant material(s) used for the purposes of extracting CBD are considered hemp, or considered marijuana.
Any psychoactive marijuana, regardless of its CBD content, is derived from the flower (or bud) of the genus cannabis. Non-psychoactive hemp (also commonly-termed industrial hemp), regardless of its CBD content, is any part of the genus cannabis plant, whether growing or not, containing a ∆-9 tetrahydrocannabinol concentration of no more than three-tenths of one percent (0.3%) on a dry weight basis. Certain standards are required for the legal growth and production of hemp. The Colorado Industrial Hemp Program registers growers of industrial hemp and samples crops to verify that the THC concentration does not exceed 0.3% on dry weight basis.[57]
With Charlotte's Web bringing about increased demand for CBD-dominant cannabis, cultivating hemp has captured the attention of U.S. farmers looking to replace dwindling tobacco-growing revenues with renewed hemp "cash crops." In Kentucky, farmers are spurred on by the Industrial Hemp Research Program, established by James Comer, commissioner of agriculture. With backing from Senator Rand Paul, Comer’s legislation[58] created regulations for farmers to legally grow hemp.[59]
Joel Stanley, CEO of Stanley Brothers Social Enterprises, said they plan to invest at least $500,000 to grow therapeutic hemp in Kentucky for their Charlotte's Web cannabidiol oil, saying, "We want to make Charlotte's Web a Kentucky Proud product."[60] In addition, Paul and Comer were able to get a provision added to the federal Farm Bill that legalized hemp production in states like Kentucky to grow the crop. The Agricultural Act of 2014 was signed by President Obama.[61]
During recent years, there has been considerable legislative activity throughout the United States with respect to legalizing the agricultural production of industrial hemp. To date, approximately 11 states have legalized industrial hemp production, including: California, Colorado, Indiana, Maine, Montana, North Dakota, Oregon, South Carolina, North Carolina, Vermont, West Virginia, and Tennessee. Many other states have passed legislation authorizing the cultivation of industrial hemp for pilot projects or studies, including: Connecticut, Delaware, Hawaii, Illinois, Kentucky, Nebraska, and Utah. Additionally, the National Association of State Departments of Agriculture and the National Conference of State Legislatures have both adopted resolutions supporting revisions to the federal rules and regulations authorizing commercial production of industrial hemp.[62][63]
The United States Drug Enforcement Administration, the DEA, recently eased some of the regulatory requirements for those who are conducting FDA-approved clinical trials on cannabidiol (CBD).[64]
Several industrial hemp varieties can be legally cultivated in western Europe. A variety such as "Fedora 17" has a cannabinoid profile consistently around 1% cannabidiol (CBD) with THC less than 0.1%.[65]
Australia
Prescription Medicine (Schedule 4) for therapeutic use containing 2 per cent (2.0%) or less of other cannabinoids commonly found in cannabis (such as ∆9-THC).[66]
Canada
Cannabidiol is a Schedule II drug in Canada. As such, it is only available with a prescription.[67]
UK
Cannabidiol, in an oral-mucosal spray formulation combined with delta-9-tetrahydrocannabinol, is a prescription product available for relief of severe spasticity due to multiple sclerosis (where other anti-spasmodics have not been effective).[68] Cannabidiol produced, sold or imported for sale or recreational use would usually be illegal under the Psychoactive Substances Act 2016.
EU
Cannabidiol is listed in EU Cosmetics Ingredient Database.[69]
References
- ↑ DEA News Release, DEA Eases Requirements for FDA Approved Clinical Trials on Cannabidiol (December 23, 2015) ("CBD is a Schedule I controlled substance as defined under the CSA."), http://www.dea.gov/divisions/hq/2015/hq122315.shtml; Joseph T. Rannazzisi Deputy Assistant Administrator Drug Enforcement Administration Before the Caucus on International Narcotics Control, United States Senate, at 2 (June 24, 2015) (CBD is a Schedule I drug.); see also Frank Robison, Elvira Strehle-Henson, Cannabis Laws and Research at Colorado Institutions of Higher Education, COLO. LAW., OCTOBER 2015, AT 73, 76 ("[T]the DEA's position on CBD is clear—it is a Schedule I substance.").
- 1 2 Mechoulam R, Parker LA, Gallily R (November 2002). "Cannabidiol: an overview of some pharmacological aspects". J Clin Pharmacol (Review). 42 (11 Suppl): 11S–19S. doi:10.1177/0091270002238789. PMID 12412831.
- ↑ Scuderi C, Filippis DD, Iuvone T, Blasio A, Steardo A, Esposito G (May 2009). "Cannabidiol in medicine: a review of its therapeutic potential in CNS disorders". Phytother Res (Review). 23 (5): 597–602. doi:10.1002/ptr.2625. PMID 18844286.
- ↑ McPartland JM, Russo EB (2001). "Cannabis and cannabis extracts: greater than the sum of their parts?" (PDF). Journal of Cannabis Therapeutics. 1 (3/4): 103–132. doi:10.1300/J175v01n03_08.
- ↑ "International Nonproprietary Names for Pharmaceutical Substances (INN)" (PDF). WHO Drug Information. 30 (2): 241. 2016.
- ↑ Borgelt LM, Franson KL, Nussbaum AM, Wang GS (February 2013). "The pharmacologic and clinical effects of medical cannabis". Pharmacotherapy (Review). 33 (2): 195–209. doi:10.1002/phar.1187. PMID 23386598.
- ↑ Aizpurua-Olaizola, Oier; Soydaner, Umut; Öztürk, Ekin; Schibano, Daniele; Simsir, Yilmaz; Navarro, Patricia; Etxebarria, Nestor; Usobiaga, Aresatz (February 2, 2016). "Evolution of the Cannabinoid and Terpene Content during the Growth ofCannabis sativaPlants from Different Chemotypes". Journal of Natural Products. 79 (2): 324–331. doi:10.1021/acs.jnatprod.5b00949.
- 1 2 3 Campos AC, Moreira FA, Gomes FV, Del Bel EA, Guimarães FS (December 2012). "Multiple mechanisms involved in the large-spectrum therapeutic potential of cannabidiol in psychiatric disorders". Philos. Trans. R. Soc. Lond., B, Biol. Sci. (Review). 367 (1607): 3364–78. doi:10.1098/rstb.2011.0389. PMC 3481531
 . PMID 23108553.
. PMID 23108553. - ↑ "What is Dravet Syndrome?". Dravetfoundation.org. June 20, 2014. Retrieved December 4, 2016.
- 1 2 Melville, Nancy A. (August 14, 2013), Seizure Disorders Enter Medical Marijuana Debate, Medscape Medical News., retrieved January 14, 2014
- ↑ Maa, Edward; Figi, Paige (2014). "The case for medical marijuana in epilepsy". Epilepsia. 55 (6): 783–786. doi:10.1111/epi.12610. ISSN 0013-9580.
- ↑ Young, Saundra. "Marijuana stops child's severe seizures" (PDF). CNN. CNN. Retrieved January 7, 2016.
- ↑ Throckmorton, Douglas (June 24, 2015). "Cannabidiol: Barriers to Research and Potential Medical Benefits". FDA. FDA. Retrieved December 15, 2015.
- ↑ Gloss D, Vickrey B (June 13, 2012). "Cannabinoids for epilepsy". Cochrane Database Syst Rev (Review). 6 (6): CD009270. doi:10.1002/14651858.CD009270.pub2. PMID 22696383.
- ↑ Devinsky, Orrin (2015). "Efficacy and Safety of Epidiolex (Cannabidiol) in Children and Young Adults with Treatment-Resistant Epilepsy". Annual Meeting Abstracts. American Epilepsy Society. Retrieved December 13, 2015.
- ↑ Angus, Chen (December 8, 2015). "Marijuana's Main Ingredient, Cannabidiol, May Be An Effective Way To Treat Epilepsy". Medical Daily. Retrieved December 14, 2015.
- ↑ Devinsky O, Cilio MR, Cross H, Fernandez-Ruiz J, French J, Hill C, Katz R, Di Marzo V, Jutras-Aswad D, Notcutt WG, Martinez-Orgado J, Robson PJ, Rohrback BG, Thiele E, Whalley B, Friedman D (2014). "Cannabidiol: pharmacology and potential therapeutic role in epilepsy and other neuropsychiatric disorders". Epilepsia (Review). 55 (6): 791–802. doi:10.1111/epi.12631. PMC 4707667
 . PMID 24854329.
. PMID 24854329. - ↑ Friedman D, Devinsky O (2016). "Cannabinoids in the Treatment of Epilepsy". N. Engl. J. Med. 374 (1): 94–5. doi:10.1056/nejmc1512758. PMID 26672645.
- ↑ Leweke FM, Mueller JK, Lange B, Rohleder C (2016). "Therapeutic Potential of Cannabinoids in Psychosis". Biol. Psychiatry. 79 (7): 604–12. doi:10.1016/j.biopsych.2015.11.018. PMID 26852073.
- ↑ Schubart CD; et al. (2013). "Cannabidiol as a potential treatment for psychosis". European Neuropsychopharmacology. 24 (1): 51–64. doi:10.1016/j.euroneuro.2013.11.002. PMID 24309088.
- ↑ Devinsky O; et al. (Jun 2014). "Cannabidiol: pharmacology and potential therapeutic role in epilepsy and other neuropsychiatric disorders". Epilepsia. 55 (6): 791–802. doi:10.1111/epi.12631. PMC 4707667
 . PMID 24854329.
. PMID 24854329. - ↑ Mechoulam R, Peters M, Murillo-Rodriguez E, Hanus LO (August 2007). "Cannabidiol—recent advances". Chem. Biodivers. (Review). 4 (8): 1678–92. doi:10.1002/cbdv.200790147. PMID 17712814.
- ↑ Pertwee RG (2008). "The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: Δ9-tetrahydrocannabinol, cannabidiol and Δ9-tetrahydrocannabivarin". British Journal of Pharmacology. 153 (2): 199–215. doi:10.1038/sj.bjp.0707442. PMC 2219532
 . PMID 17828291.
. PMID 17828291. - ↑ Hayakawa K, Mishima K, Hazekawa M, Sano K, Irie K, Orito K, Egawa T, Kitamura Y, Uchida N, Nishimura R, Egashira N, Iwasaki K, Fujiwara M (2008). "Cannabidiol potentiates pharmacological effects of Δ9-tetrahydrocannabinol via CB1 receptor-dependent mechanism". Brain Research. 1188: 157–164. doi:10.1016/j.brainres.2007.09.090. PMID 18021759.
- ↑ Alchimia Blog, Cannabinoids and their medicinal properties
- ↑ Ryberg E, Larsson N, Sjögren S, Hjorth S, Hermansson NO, Leonova J, Elebring T, Nilsson K, Drmota T, Greasley PJ (2007). "The orphan receptor GPR55 is a novel cannabinoid receptor". British Journal of Pharmacology. 152 (7): 1092–101. doi:10.1038/sj.bjp.0707460. PMC 2095107
 . PMID 17876302.
. PMID 17876302. - ↑ Russo EB, Burnett A, Hall B, Parker KK (August 2005). "Agonistic properties of cannabidiol at 5-HT1a receptors". Neurochemical Research. 30 (8): 1037–43. doi:10.1007/s11064-005-6978-1. PMID 16258853.
- ↑ Zanelati TV, Biojone C, Moreira FA, Guimarães FS, Joca SR (January 2010). "Antidepressant-like effects of cannabidiol in mice: possible involvement of 5-HT1A receptors". British Journal of Pharmacology. 159 (1): 122–8. doi:10.1111/j.1476-5381.2009.00521.x. PMC 2823358
 . PMID 20002102.
. PMID 20002102. - 1 2 Resstel LB, Tavares RF, Lisboa SF, Joca SR, Corrêa FM, Guimarães FS (January 2009). "5-HT1A receptors are involved in the cannabidiol-induced attenuation of behavioural and cardiovascular responses to acute restraint stress in rats". British Journal of Pharmacology. 156 (1): 181–8. doi:10.1111/j.1476-5381.2008.00046.x. PMC 2697769
 . PMID 19133999.
. PMID 19133999. - ↑ Campos AC, Guimarães FS (August 2008). "Involvement of 5HT1A receptors in the anxiolytic-like effects of cannabidiol injected into the dorsolateral periaqueductal gray of rats". Psychopharmacology. 199 (2): 223–30. doi:10.1007/s00213-008-1168-x. PMID 18446323.
- ↑ Mishima K, Hayakawa K, Abe K, Ikeda T, Egashira N, Iwasaki K, Fujiwara M (May 2005). "Cannabidiol prevents cerebral infarction via a serotonergic 5-hydroxytryptamine1A receptor-dependent mechanism". Stroke; a Journal of Cerebral Circulation. 36 (5): 1077–82. doi:10.1161/01.STR.0000163083.59201.34. PMID 15845890.
- ↑ Hayakawa K, Mishima K, Nozako M, Ogata A, Hazekawa M, Liu AX, Fujioka M, Abe K, Hasebe N, Egashira N, Iwasaki K, Fujiwara M (March 2007). "Repeated treatment with cannabidiol but not Delta9-tetrahydrocannabinol has a neuroprotective effect without the development of tolerance". Neuropharmacology. 52 (4): 1079–87. doi:10.1016/j.neuropharm.2006.11.005. PMID 17320118.
- ↑ Kathmann M, Flau K, Redmer A, Tränkle C, Schlicker E (2006). "Cannabidiol is an allosteric modulator at mu- and delta-opioid receptors". Naunyn-Schmiedeberg's Archives of Pharmacology. 372 (5): 354–361. doi:10.1007/s00210-006-0033-x. PMID 16489449.
- ↑ Ujváry I, Hanus L (2014). "Human metabolites of cannabidiol: a review on their formation, biological activity, and relevance in therapy". Cannabis and Cannabinoid Research. 1 (1): 90–101. doi:10.1089/can.2015.0012.
- ↑ Bornheim LM, Kim KY, Li J, Perotti BY, Benet LZ (August 1995). "Effect of cannabidiol pretreatment on the kinetics of tetrahydrocannabinol metabolites in mouse brain". Drug Metabolism and Disposition. 23 (8): 825–831. PMID 7493549.
- ↑ Klein C, Karanges E, Spiro A, Wong A, Spencer J, Huynh T, Gunasekaran N, Karl T, Long LE, Huang XF, Liu K, Arnold JC, McGregor IS (November 2011). "Cannabidiol potentiates Δ⁹-tetrahydrocannabinol (THC) behavioural effects and alters THC pharmacokinetics during acute and chronic treatment in adolescent rats". Psychopharmacology. 218 (2): 443–457. doi:10.1007/s00213-011-2342-0. PMID 21667074.
- ↑ Hunt CA, Jones RT, Herning RI, Bachman J (June 1981). "Evidence that Cannabidiol Does Not Significantly Alter the Pharmacokinetics of Tetrahydrocannabinol in Man". Journal of Pharmacokinetics and Biopharmaceutics. 9 (3): 245–260. doi:10.1007/BF01059266. PMID 6270295.
- ↑ United States Adopted Names Council: Statement on a nonproprietary name
- ↑ "Fact Sheet — Sativex". Health Canada. Retrieved May 16, 2013.
- ↑ "Cannabis-Derived Dravet Syndrome Drug Gets US Orphan Drug Approval". November 18, 2013. Retrieved July 21, 2015.
- ↑ Torres, Kristina (September 30, 2015). "Georgia doctors encouraged in study of medical marijuana". The Atlanta Journal.
- ↑ Jones PG, Falvello L, Kennard O, Sheldrick GM, Mechoulam R (1977). "Cannabidiol". Acta Crystallogr. B. 33 (10): 3211–3214. doi:10.1107/S0567740877010577.
- ↑ Mechoulam R, Ben-Zvi Z, Gaoni Y (1968). "Hashish—XIII On the nature of the beam test". Tetrahedron. 24 (16): 5615–5624. doi:10.1016/0040-4020(68)88159-1. PMID 5732891.
- ↑ Gaoni Y, Mechoulam R (1966). "Hashish—VII The isomerization of cannabidiol to tetrahydrocannabinols". Tetrahedron. 22 (4): 1481–1488. doi:10.1016/S0040-4020(01)99446-3.
- ↑ Petrzilka T, Haefliger W, Sikemeier C, Ohloff G, Eschenmoser A (1967). "Synthese und Chiralität des (−)-Cannabidiols". Helv. Chim. Acta. 50 (2): 719–723. doi:10.1002/hlca.19670500235. PMID 5587099.
- ↑ Gaoni Y, Mechoulam R (1985). "Boron trifluoride etherate on alumuna — a modified Lewis acid reagent. An improved synthesis of cannabidiol". Tetrahedron Letters. 26 (8): 1083–1086. doi:10.1016/S0040-4039(00)98518-6.
- ↑ Kobayashi Y, Takeuchi A, Wang YG (2006). "Synthesis of cannabidiols via alkenylation of cyclohexenyl monoacetate". Org. Lett. 8 (13): 2699–2702. doi:10.1021/ol060692h. PMID 16774235.
- ↑ Marks MD, Tian L, Wenger JP, Omburo SN, Soto-Fuentes W, He J, Gang DR, Weiblen GD, Dixon RA (2009). "Identification of candidate genes affecting Δ9-tetrahydrocannabinol biosynthesis in Cannabis sativa". Journal of Experimental Botany. 60 (13): 3715–3726. doi:10.1093/jxb/erp210. PMC 2736886
 . PMID 19581347.
. PMID 19581347. - ↑ Taura, F., Sirikantaramas, S., Shoyama, Y., Yoshikai, K., Shoyama, Y., Morimoto, S. (2007). "Cannabidiolic-acid synthase, the chemotype-determining enzyme in the fiber-type Cannabis sativa". FEBS Letters. 581 (16): 2929–34. doi:10.1016/j.febslet.2007.05.043. PMID 17544411.
- ↑ Romney, Lee (September 13, 2012). "On the frontier of medical pot to treat boy's epilepsy". Los Angeles Times.
- ↑ Good, Alastair (October 26, 2010). "Growing marijuana that won't get you high". The Daily Telegraph. London.
- ↑ Sachs J; et al. (Oct 2015). "Safety and Toxicology of Cannabinoids". Neurotherapeutics. 12 (4): 735–746. doi:10.1007/s13311-015-0380-8. PMC 4604177
 . PMID 26269228.
. PMID 26269228. - ↑ Iseger TA, Bossong MG (2015). "A systematic review of the antipsychotic properties of cannabidiol in humans". Schizophr. Res. 162 (1–3): 153–61. doi:10.1016/j.schres.2015.01.033. PMID 25667194.
- ↑ Sachs J et al Safety and Toxicology of Cannabinoids. Neurotherapeutics. 2015 Oct; 12(4): 735–746. PMC4604177
- ↑ Izzo AA, Borrelli F, Capasso R, Di Marzo V, Mechoulam R (2009). "Non-psychotropic plant cannabinoids: new therapeutic opportunities from an ancient herb". Trends Pharmacol. Sci. 30 (10): 515–27. doi:10.1016/j.tips.2009.07.006. PMID 19729208.
- ↑ Izzo AA, Borrelli F, Capasso R, Di Marzo V, Mechoulam R (2009). "Non-psychotropic plant cannabinoids: new therapeutic opportunities from an ancient herb" (PDF). Trends Pharmacol. Sci. 30 (10): 515–27. doi:10.1016/j.tips.2009.07.006. PMID 19729208.
- ↑ "Industrial Hemp | Department of Agriculture – Plants". Colorado.gov. Retrieved December 4, 2016.
- ↑ Lord, Joseph (January 28, 2013). "Kentucky Hemp Commission Supports Senate Bill; James Comer Responds to Critics | 89.3 WFPL News Louisville". Wfpl.org. Retrieved December 4, 2016.
- ↑ Firger, Jessica (October 23, 2015). "The Great Kentucky Hemp Experiment". Newsweek.com. Retrieved December 4, 2016.
- ↑ Patton, Janet (May 5, 2015). "Hemp industry is growing in Kentucky, attracting processors, investment | Lexington Herald-Leader". Kentucky.com. Retrieved December 4, 2016.
- ↑ Shear, Michael D. (February 7, 2014) In Signing Farm Bill, Obama Extols Rural Growth. New York Times.
- ↑ HOUSE BILL REPORT SSB 5012. leg.wa.gov
- ↑ State Industrial Hemp Statutes. U.S. National Conference of State Legislatures (August 19, 2016)
- ↑ "Headquarters News Releases, 12/23/15". Dea.gov. December 23, 2015. Retrieved December 4, 2016.
- ↑ Fournier, G.; Beherec, O.; Bertucelli, S. (2003). "Intérêt du rapport Δ-9-THC / CBD dans le contrôle des cultures de chanvre industriel". Annales de Toxicologie Analytique. 15 (4): 250–259. doi:10.1051/ata/2003003.
- ↑ "Poisons Standard March 2016". Legislation.gov.au. Retrieved December 4, 2016.
- ↑ "Controlled Drugs and Substances Act – Schedule II". Laws-lois.justice.gc.ca. Retrieved December 4, 2016.
- ↑ "Sativex Oromucosal Spray – Summary of Product Characteristics (SPC) – (eMC)". Medicines.org.uk. Retrieved December 4, 2016.
- ↑ "CosIng – Cosmetics – GROWTH – European Commission". Ec.europa.eu. Retrieved December 4, 2016.
External links
- Project CBD Non-profit educational service dedicated to promoting and publicizing research into the medical utility of cannabidiol.
