Bismuthine
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
bismuthane | |||
| Other names | |||
| Identifiers | |||
| 18288-22-7 | |||
| 3D model (Jmol) | Interactive image | ||
| ChemSpider | 8886 | ||
| PubChem | 9242 | ||
| |||
| |||
| Properties | |||
| BiH3 | |||
| Molar mass | 212.00 g/mol | ||
| Appearance | colourless gas | ||
| Density | 0.008665 g/mL (20 °C) | ||
| Boiling point | 16.8 °C (62.2 °F; 289.9 K) (extrapolated) | ||
| Structure | |||
| trigonal pyramidal | |||
| Related compounds | |||
| Related hydrides |
Ammonia Phosphine Arsine Stibine | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| | |||
| Infobox references | |||
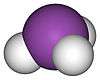
Bismuthine (IUPAC name: bismuthane) is the chemical compound with the formula BiH3. As the heaviest analogue of ammonia (a pnictogen hydride), BiH3 is unstable, decomposing to bismuth metal well below 0 °C. This compound adopts the expected pyramidal structure with H-Bi-H angles of around 90°.[1]
The term bismuthine may also refer to a member of the family of organobismuth(III) species having the general formula BiR
3, where R is an organic substituent. For example, Bi(CH3)3 is trimethylbismuthine.
Preparation and properties
BiH3 is prepared by the redistribution of methylbismuthine (BiH2Me):[2]
- 3 BiH2Me → 2 BiH3 + BiMe3
The required BiH2Me, which is also thermally unstable, is generated by reduction of methylbismuth dichloride, BiCl2Me with LiAlH4.[1]
As suggested by the behavior of SbH3, BiH3 is unstable and decomposes to its constituent elements according to the following equation:
- 2 BiH3 → 3 H2 + 2 Bi (ΔHf'
ogas = −278 kJ/mol)
The methodology used for detection of arsenic ("Marsh test") can also be used to detect BiH3. This test relies on the thermal decomposition of these trihydrides to the metallic mirrors of reduced As, Sb, and Bi. These deposits can be further distinguished by their distinctive solubility characteristics: arsenic dissolves in NaOCl, antimony dissolves in ammonium polysulfide, and bismuth resists both reagents.[2]
Uses and safety considerations
The low stability of BiH3 poses significant hazards and precludes technical applications, except as an intermediary product.
References
- 1 2 W. Jerzembeck; H. Bürger; L. Constantin; L. Margulès; J. Demaison; J. Breidung; W. Thiel (2002). "Bismuthine BiH3: Fact or Fiction? High-Resolution Infrared, Millimeter-Wave, and Ab Initio Studies". Angew. Chem. Int. Ed. 41 (14): 2550–2552. doi:10.1002/1521-3773(20020715)41:14<2550::AID-ANIE2550>3.0.CO;2-B.
- 1 2 Holleman, A. F.; Wiberg, E. "Inorganic Chemistry" Academic Press: San Diego, 2001.ISBN 0-12-352651-5.