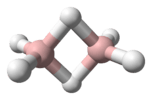Diphosphane
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Diphosphane | |||
| Systematic IUPAC name
Diphosphane (substitutive) Tetrahydidodiphosphorus(P—P) (additive) | |||
| Other names
Diphosphine | |||
| Identifiers | |||
| 13445-50-6 | |||
| 3D model (Jmol) | Interactive image | ||
| ChEBI | CHEBI:35880 | ||
| ChemSpider | 122832 | ||
| PubChem | 139283 | ||
| |||
| |||
| Properties | |||
| H4P2 | |||
| Molar mass | 65.98 g·mol−1 | ||
| Melting point | −99 °C (−146 °F; 174 K) | ||
| Boiling point | 63.5 °C (146.3 °F; 336.6 K) (Extrapolated, decomposes) | ||
| Related compounds | |||
| Other anions |
ammonia hydrazine triazane | ||
| Other cations |
diphosphines | ||
| Related Binary Phosphorus halides |
diphosphorus tetrafluoride diphosphorus tetrachloride diphosphorus tetrabromide diphosphorus tetraiodide | ||
| Related compounds |
phosphane triphosphane diphosphene diphosphenes | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| | |||
| Infobox references | |||
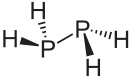
Diphosphane is an inorganic compound with the chemical formula P2H4. This colourless liquid is one of several binary phosphorus hydrides. It is the impurity that typically causes samples of phosphine to ignite in air. An older name is diphosphine.
Properties, preparation, reactions
Diphosphane adopts the gauche conformation (like hydrazine, less symmetrical than shown in the image) with a P-P distance of 2.219 angstroms. It is nonbasic, unstable at room temperature, and spontaneously flammable in air. It is only poorly soluble in water but dissolves in organic solvents. Its 1H NMR spectrum consists of 32 lines resulting from an A2XX'A'2 splitting system.[1]
Diphosphane is produced by the hydrolysis of calcium monophosphide, which can be described as the Ca2+ derivative of P24−. According to an optimized procedure, hydrolysis of 400 g of CaP at -30 °C gives about 20 g of product, slightly contaminated with phosphine.
Reaction of diphosphine with butyllithium affords a variety of condensed polyphosphine compounds.
Organic diphosphanes
A variety of organic derivatives of diphosphane are known. These species are prepared by reductive coupling, e.g. from chlorodiphenylphosphine:
- 2ClPPh2 + 2 Na → Ph2PPPh2 + 2 NaCl
The methyl compound P2Me4 is prepared by the reaction of thiophosphoryl chloride with methylmagnesium bromide.
See also
References
- ↑ Marianne Baudler, Klaus Glinka (1993). "Monocyclic and polycyclic phosphines". Chem. Rev. 93 (4): 1623–1667. doi:10.1021/cr00020a010.