G-130
This article is about the stimulant drug. For the glycoprotein, see Gp130.
 | |
| Identifiers | |
|---|---|
| |
| CAS Number |
42013-48-9 |
| PubChem (CID) | 97711 |
| ChemSpider | 88190 |
| Chemical and physical data | |
| Formula | C12H17NO |
| Molar mass | 191.269 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
| (verify) | |
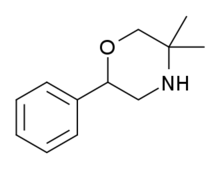
G-130 (GP-130, 2-Phenyl-5,5-dimethyltetrahydro-1,4-oxazine)[1] is a drug with stimulant and anorectic effects, related to phenmetrazine.[2][3][4]
Synthesis
It is made by the dehydration of depradinol with conc sulphuric acid.
Structural analogs
Compounds related to G 130 and radafaxine were synthesized that behave as combined inhibitors of monoamine uptake and nAChRs.[5]
See also
- 2-Phenyl-3,6-dimethylmorpholine
- 3-Fluorophenmetrazine
- Phendimetrazine (2-Phenyl-3,4-dimethylmorpholine)
- Manifaxine
- Radafaxine
References
- ↑ GB 1336732 - Diethanolamine Derivatives.
- ↑ Fanelli O (June 1973). "Pharmacological and toxicological study of a new psychotropic stimulant: the 2-phenyl-5-dimethyl-tetrahydro-1,4-oxazine, in comparison with d,l-amphetamine, phenmetrazine and pemoline-Mg". Arzneimittel-Forschung. 23 (6): 810–6. PMID 4740767.
- ↑ Fanelli O, Mazzoncini V, Ferri S (October 1974). "Toxicological and teratological study of 2-phenyl-5,5-dimethyl-tetrahydro-1,4-oxazine hydrochloride (G 130), a psychostimulant drug". Arzneimittel-Forschung. 24 (10): 1627–32. PMID 4479774.
- ↑ Fanelli O, Mazzoncini V, Trallori L (December 1974). "Antagonism of psychostimulant 2-phenyl-5,5-dimethyl-tetrahydro-1,4-oxazine hydrochloride (G 130) to central nervous system depressing drugs. Monoamine oxidase inhibitory activity and norepinephrine and serotonin induced changes. Comparison with dL-amphetamine". Arzneimittel-Forschung. 24 (12): 2025–9. PMID 4480283.
- ↑ Carroll, F. I.; Muresan, A. Z.; Blough, B. E.; Navarro, H. N. A.; Mascarella, S. W.; Eaton, J. B.; Huang, X.; Damaj, M. I.; Lukas, R. J. (2011). "Synthesis of 2-(Substituted Phenyl)-3,5,5-trimethylmorpholine Analogues and Their Effects on Monoamine Uptake, Nicotinic Acetylcholine Receptor Function, and Behavioral Effects of Nicotine". Journal of Medicinal Chemistry. 54 (5): 1441–1448. doi:10.1021/jm1014555. PMC 3048909
 . PMID 21319801.
. PMID 21319801.
This article is issued from Wikipedia - version of the 11/12/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.