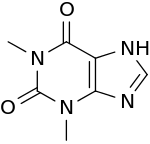
Theophylline
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Theolair, Slo-Bid |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a681006 |
| Pregnancy category | |
| Routes of administration | oral, IV, rectal |
| ATC code | R03DA04 (WHO) |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 100% |
| Protein binding | 40%, primarily to albumin |
| Metabolism | hepatic to 1-methyluric acid |
| Biological half-life | 5–8 hours |
| Identifiers | |
| |
| CAS Number |
58-55-9 |
| PubChem (CID) | 2153 |
| IUPHAR/BPS | 413 |
| DrugBank |
DB00277 |
| ChemSpider |
2068 |
| UNII |
0I55128JYK |
| KEGG |
D00371 |
| ChEBI |
CHEBI:28177 |
| ChEMBL |
CHEMBL190 |
| ECHA InfoCard | 100.000.350 |
| Chemical and physical data | |
| Formula | C7H8N4O2 |
| Molar mass | 180.164 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
| (verify) | |

Theophylline, also known as 1,3-dimethylxanthine, is a methylxanthine drug used in therapy for respiratory diseases such as chronic obstructive pulmonary disease (COPD) and asthma under a variety of brand names. As a member of the xanthine family, it bears structural and pharmacological similarity to theobromine and caffeine. A small amount of theophylline is one of the products of caffeine metabolic processing in the liver.[1]
Medical uses
The main actions of theophylline involve:
- relaxing bronchial smooth muscle
- increasing heart muscle contractility and efficiency; as a positive inotropic
- increasing heart rate: (positive chronotropic)[2]
- increasing blood pressure
- increasing renal blood flow
- anti-inflammatory effects
- central nervous system stimulatory effect mainly on the medullary respiratory center.
The main therapeutic uses of theophylline are aimed at:
- chronic obstructive pulmonary disease (COPD)
- asthma
- infant apnea
- Blocks the action of adenosine, an inhibitory neurotransmitter that induces sleep, contracts the smooth muscles and relaxes the cardiac muscle.
Uses under investigation
A clinical study reported in 2008 that theophylline was helpful in improving the sense of smell in study subjects with anosmia.[3]
Adverse effects
The use of theophylline is complicated by its interaction with various drugs, chiefly as cimetidine and phenytoin, and that it has a narrow therapeutic index, so its use must be monitored by direct measurement of serum theophylline levels to avoid toxicity. It can also cause nausea, diarrhea, increase in heart rate, abnormal heart rhythms, and CNS excitation (headaches, insomnia, irritability, dizziness and lightheadedness).[4][5] Seizures can also occur in severe cases of toxicity and is considered to be a neurological emergency.[6] Its toxicity is increased by erythromycin, cimetidine, and fluoroquinolones, such as ciprofloxacin. It can reach toxic levels when taken with fatty meals, an effect called dose dumping.[7] Theophylline toxicity can be treated with beta blockers. In addition to seizures, tachyarrhythmias are a major concern.[8]
Mechanisms of action
Like other methylated xanthine derivatives, theophylline is both a
- competitive nonselective phosphodiesterase inhibitor,[9] which raises intracellular cAMP, activates PKA, inhibits TNF-alpha[10][11] and inhibits leukotriene[12] synthesis, and reduces inflammation and innate immunity[12]
- nonselective adenosine receptor antagonist,[13] antagonizing A1, A2, and A3 receptors almost equally, which explains many of its cardiac effects
Theophylline has been shown to inhibit TGF-beta-mediated conversion of pulmonary fibroblasts into myofibroblasts in COPD and asthma via cAMP-PKA pathway and suppresses COL1 mRNA, which codes for the protein collagen.[14]
It has been shown that theophylline may reverse the clinical observations of steroid insensitivity in patients with COPD and asthmatics that are active smokers (a condition resulting in oxidative stress) via a distinctly separate mechanism. Theophylline in vitro can restore the reduced HDAC (histone deacetylase) activity that is induced by oxidative stress (i.e., in smokers), returning steroid responsiveness toward normal.[15] Furthermore, theophylline has been shown to directly activate HDAC2.[15] (Corticosteroids switch off the inflammatory response by blocking the expression of inflammatory mediators through deacetylation of histones, an effect mediated via histone deacetylase-2 (HDAC2). Once deacetylated, DNA is repackaged so that the promoter regions of inflammatory genes are unavailable for binding of transcription factors such as NF-κB that act to turn on inflammatory activity. It has recently been shown that the oxidative stress associated with cigarette smoke can inhibit the activity of HDAC2, thereby blocking the anti-inflammatory effects of corticosteroids.)
Natural occurrences
Theophylline is naturally found in cocoa beans. Amounts as high as 3.7 mg/g have been reported in Criollo cocoa beans.[16]
Trace amounts of theophylline are also found in brewed tea, although brewed tea provides only about 1 mg/L,[17] which is significantly less than a therapeutic dose.
Pharmacokinetics
Absorption
When theophylline is administered intravenously, bioavailability is 100%, as with all intravenously administered drugs.
Distribution
Theophylline is distributed in the extracellular fluid, in the placenta, in the mother's milk and in the central nervous system. The volume of distribution is 0.5 L/kg. The protein binding is 40%. The volume of distribution may increase in neonates and those suffering from cirrhosis or malnutrition, whereas the volume of distribution may decrease in those who are obese.
Metabolism
Theophylline is metabolized extensively in the liver (up to 70%). It undergoes N-demethylation via cytochrome P450 1A2. It is metabolized by parallel zero order and Michaelis-Menten pathways. Metabolism may become saturated (non-linear), even within the therapeutic range. Small dose increases may result in disproportionately large increases in serum concentration. Methylation to caffeine is also important in the infant population. Smokers and people with hepatic (liver) impairment metabolize it differently. Both THC and nicotine have been shown to increase the rate of theophylline metabolism.[18]
Elimination
Theophylline is excreted unchanged in the urine (up to 10%). Clearance of the drug is increased in these conditions: children 1 to 12, teenagers 12 to 16, adult smokers, elderly smokers, cystic fibrosis, hyperthyroidism. Clearance of the drug is decreased in these conditions: elderly, acute congestive heart failure, cirrhosis, hypothyroidism and febrile viral illness.
The elimination half-life varies: 30 hours for premature neonates, 24 hours for neonates, 3.5 hours for children ages 1 to 9, 8 hours for adult non-smokers, 5 hours for adult smokers, 24 hours for those with hepatic impairment, 12 hours for those with congestive heart failure NYHA class I-II, 24 hours for those with congestive heart failure NYHA class III-IV, 12 hours for the elderly.
History
Theophylline was first extracted from tea leaves and chemically identified around 1888 by the German biologist Albrecht Kossel.[19][20] Just seven years after its discovery, a chemical synthesis starting with 1,3-dimethyluric acid was described by Emil Fischer and Lorenz Ach.[21] The Traube purine synthesis, an alternative method to synthesize theophylline, was introduced in 1900 by another German scientist, Wilhelm Traube.[22] Theophylline's first clinical use came in 1902 as a diuretic.[23] It took an additional 20 years until its first description in asthma treatment.[24] The drug was prescribed in a liquid syrup by the 1970s as Theostat 20 and Theostat 80, and by the early 1980s in a tablet form called Quibron.
References
- ↑ Mandal, Ananya. "Caffeine Pharmacology". Website Medical News.
- ↑ Alboni et al. Effects of Permanent Pacemaker and Oral Theophylline in Sick Sinus Syndrome The THEOPACE Study: A Randomized Controlled Trial
- ↑ For Some Who Have Lost Their Sense Of Smell, A Once Popular Asthma Drug Could Help. Science Daily, American Physiological Society
- ↑ MedlinePlus Drug Information: Theophylline
- ↑ THEOPHYLLINE - ORAL 24 HOUR TABLET (Uni-Dur) side effects, medical uses, and drug interactions
- ↑ Yoshikawa H (Apr 2007). "First-line therapy for theophylline-associated seizures.". Acta Neurol Scand. 115 (4 Suppl): 57–61. doi:10.1111/j.1600-0404.2007.00810.x. PMID 17362277.
- ↑ Hendeles L, Weinberger M, Milavetz G, Hill M, Vaughan L (1985). "Food-induced "dose-dumping" from a once-a-day theophylline product as a cause of theophylline toxicity". Chest. 87 (6): 758–65. doi:10.1378/chest.87.6.758. PMID 3996063.
- ↑ Seneff M, Scott J, Friedman B, Smith M (1990). "Acute theophylline toxicity and the use of esmolol to reverse cardiovascular instability". Annals of Emergency Medicine. 19 (6): 671–3. doi:10.1016/s0196-0644(05)82474-6. PMID 1971502.
- ↑ Essayan DM (2001). "Cyclic nucleotide phosphodiesterases". J Allergy Clin Immunol. 108 (5): 671–80. doi:10.1067/mai.2001.119555. PMID 11692087.
- ↑ Deree J, Martins JO, Melbostad H, Loomis WH, Coimbra R (2008). "Insights into the Regulation of TNF-α Production in Human Mononuclear Cells: The Effects of Non-Specific Phosphodiesterase Inhibition". Clinics (Sao Paulo). 63 (3): 321–8. doi:10.1590/S1807-59322008000300006. PMC 2664230
 . PMID 18568240.
. PMID 18568240. - ↑ Marques LJ, Zheng L, Poulakis N, Guzman J, Costabel U (February 1999). "Pentoxifylline inhibits TNF-alpha production from human alveolar macrophages". Am. J. Respir. Crit. Care Med. 159 (2): 508–11. doi:10.1164/ajrccm.159.2.9804085. PMID 9927365.
- 1 2 Peters-Golden M, Canetti C, Mancuso P, Coffey MJ (2005). "Leukotrienes: underappreciated mediators of innate immune responses". J Immunol. 174 (2): 589–94. doi:10.4049/jimmunol.174.2.589. PMID 15634873.
- ↑ Daly JW, Jacobson KA, Ukena D (1987). "Adenosine receptors: development of selective agonists and antagonists". Prog Clin Biol Res. 230 (1): 41–63. PMID 3588607.
- ↑ Yano Y, Yoshida M, Hoshino S, Inoue K, Kida H, Yanagita M, Takimoto T, Hirata H, Kijima T (2006). "Anti-fibrotic effects of theophylline on lung fibroblasts". Biochemical and Biophysical Research Communications. 341 (3): 684–90. doi:10.1016/j.bbrc.2006.01.018. PMID 16430859.
- 1 2 Ito K, Lim S, Caramori G, Cosio B, Chung KF, Adcock IM, Barnes PJ (2002). "A molecular mechanism of action of theophylline: Induction of histone deacetylase activity to decrease inflammatory gene expression". Proceedings of the National Academy of Sciences of the United States of America. 99 (13): 8921–6. doi:10.1073/pnas.132556899. PMC 124399
 . PMID 12070353.
. PMID 12070353. - ↑ Apgar, Joan L; Tarka, Jr., Stanly M. (1998). "Methylxanthine composition and consumption patterns of cocoa and chocolate products and their uses". In Gene A. Spiller. Caffeine. CRC Press. p. 171. ISBN 0-8493-2647-8. Retrieved 2013-11-10.
- ↑ MAFF Food Surveillance Information Sheet
- ↑ "RxList Marinol Interactions". 2008-05-29. Retrieved 2013-06-02. (accessdate may be 2013-02-06)
- ↑ Kossel A (1888). "Über eine neue Base aus dem Pflanzenreich". Ber. Dtsch. Chem. Ges. 21: 2164–2167. doi:10.1002/cber.188802101422.
- ↑ Kossel A (1889). "Über das Theophyllin, einen neuen Bestandtheil des Thees". Hoppe Seylers Z. Physiol. Chem. 13: 298–308.
- ↑ Fischer E, Ach L (1895). "Synthese des Caffeins". Ber. Dtsch. Chem. Ges. 28: 3139. doi:10.1002/cber.189502803156.
- ↑ Traube W (1900). "Der synthetische Aufbau der Harnsäure, des Xanthins, Theobromins, Theophyllins und Caffeïns aus der Cyanessigsäure]". Chem. Ber. 33 (3): 3035–3056. doi:10.1002/cber.19000330352.
- ↑ Minkowski O (1902). "Über Theocin (Theophyllin) als Diureticum". Ther. Gegenwart. 43: 490–493.
- ↑ Schultze-Werninghaus G, Meier-Sydow J (1982). "The clinical and pharmacological history of theophylline: first report on the bronchospasmolytic action in man by S. R. Hirsch in Frankfurt (Main) 1922". Clin. Allergy. 12 (2): 211–215. doi:10.1111/j.1365-2222.1982.tb01641.x. PMID 7042115.
External links
| Wikimedia Commons has media related to Theophylline. |