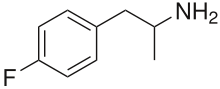
4-Fluoroamphetamine
 | |
 | |
| Clinical data | |
|---|---|
| Pregnancy category |
|
| Routes of administration | oral |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number |
459-02-9 |
| PubChem (CID) | 9986 |
| ChemSpider |
9592 |
| Chemical and physical data | |
| Formula | C9H12FN |
| Molar mass | 153.20 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
| | |
4-Fluoroamphetamine (4-FA; 4-FMP; PAL-303; "Flux"), also known as para-fluoroamphetamine (PFA) is a psychoactive research chemical of the phenethylamine and substituted amphetamine chemical classes. It produces stimulant and entactogenic effects, and is described subjectively as being between amphetamine and MDMA. As a recreational drug, 4-FA is sometimes sold along with related compounds such as 2-fluoroamphetamine and 4-fluoromethamphetamine.[1][2]
Usage
4-FA is popular in the Netherlands where it is predominantly used for its specific effects (77% of users) rather than its legal status (18%).[3]
Effects
The subjective effects of 4-fluoroamphetamine include euphoria which some find similar to the effects of MDMA and amphetamine,[3] increased energy (stimulation), mood elevation, feelings of warmth and empathy, excessive talking, bruxism, and suppressed appetite (anorexic). The general course of effects involves primarily empathogenic effects for the first few hours, which fades out as increased stimulation develops over the next several hours.
The dopamine reuptake inhibition produced by 4-FA is stronger than that of either 4-CA or 4-IA.[4] 4-FA also produces less hyperthermia than similar compounds such as PMA, 3-MTA and 4-methylamphetamine.
Common acute side effects are nausea, headaches, increased heart rate and insomnia.
Chemistry
4-FA reacts with reagent tests to give a semi-unique array of colors which can be used to aid its identification.
| Reagent | Reaction color |
|---|---|
| Marquis | No reaction[5] |
| Mandelin | Pale Blue[5][6] |
| Liebermann | Orange[5][6] |
| Froehde | Faint purple/brown[5] or no reaction. |
Pharmacology
4-Fluoroamphetamine is a releasing agent and reuptake inhibitor of dopamine, serotonin, and norepinephrine. The respective EC50 values are 2.0 x 10−7 M, 7.3 x 10−7 M, and 0.37 x 10−7 M, while the IC50 values are 7.7 x 10−7 M, 68 x 10−7 M, and 4.2 x 10−7 M.[2]
Regarding the metabolic fate of 4-FA, the C-F bond at the 4-position on the phenyl ring likely resists deactivation in the liver by cytochrome P450 oxidase.[7]
Neurotoxicity
4-FA does not cause long-lasting depletion of brain serotonin, unlike its analogs 4-CA and 4-BA.[8] This is thought to "reflect the inability of the fluoro-compound to be metabolized in the same way as the other haloamphetamines."[9]
Neurotoxicity does not increase down the series of para-halogenated amphetamine derivatives, even though serotonin releasing potency does follow this trend. For example, 4-iodoamphetamine is less toxic than is 4-chloroamphetamine.[4][10] Hence, this property is not related to serotonin releasing potency as such, since PAL-287 was reported to be not at all neurotoxic even though it is a powerful 5-HT releasing agent.[11] It is unclear where 4-methylamphetamine fits in on the neurotoxicity scale. The extensive serotonergic neurotoxicity of 4-chloroamphetamine (and its brominated derivative), and the increased serotonergic toxicity of 4-methylamphetamine[12] suggest that para-substitution seems to increase overall serotonergic (neuro)toxicity, compared to amphetamine. Exceptions include 4-MTA, a para-substituted, non-neurotoxic amphetamine.[13][14][15]
Toxicology
The LD50 (mouse; i.p.) of 4-FA is 46 mg/kg.[16]
4-FA has only been involved in one death worldwide, where it was combined with amphetamine, methadone and benzodiazepines.[17]
Legal Status
China
As of October 2015 4-FA is a controlled substance in China.[18]
Czech Republic
4-FA is banned in the Czech Republic.[19]
4 FA is also controlled in: UK, Germany, Israel, Slovakia, Chile, Brazil, Canada, Croatia and Serbia.
See also
- 2-Fluoroamphetamine (2-FA)
- 3-Fluoroamphetamine (3-FA)
- 4-Fluoromethamphetamine (4-FMA)
- 4-Fluoromethcathinone (4-FMC)
- para-Bromoamphetamine (PBA)
References
- ↑ Rösner, P; Quednow, B; Girreser, U; Junge, T (2005). "Isomeric fluoro-methoxy-phenylalkylamines: a new series of controlled-substance analogues (designer drugs)". Forensic Science International. 148 (2–3): 143–56. doi:10.1016/j.forsciint.2004.05.003. PMID 15639609.
- 1 2 Nagai, F; Nonaka, R; Satoh Hisashi Kamimura, K (2007). "The effects of non-medically used psychoactive drugs on monoamine neurotransmission in rat brain". European Journal of Pharmacology. 559 (2–3): 132–7. doi:10.1016/j.ejphar.2006.11.075. PMID 17223101.
- 1 2 Linsen, Felix; Koning, Raoul P. J.; van Laar, Margriet; Niesink, Raymond J. M.; Koeter, Maarten W.; Brunt, Tibor M. (2015). "4-Fluoroamphetamine in the Netherlands: more than a one-night stand". Addiction. 110 (7): 1138–1143. doi:10.1111/add.12932. ISSN 0965-2140.
- 1 2 Marona-Lewicka, D; Rhee, GS; Sprague, JE; Nichols, DE (1995). "Psychostimulant-like effects of p-fluoroamphetamine in the rat". European Journal of Pharmacology. 287 (2): 105–13. doi:10.1016/0014-2999(95)00478-5. PMID 8749023.
- 1 2 3 4 "4-FA reaction colour results with liebermann and froehde reagent test kits". Reagent Tests UK. 3 January 2016. Retrieved 14 February 2016.
- 1 2 Uchiyama N, Kawamura M, Kamakura H, Kikura-Hanajiri R, Goda Y (2008). "Analytical Data of Designated Substances Controlled by the Pharmaceutical Affairs Law in Japan, Part II: Color Test and TLC" (PDF). Yakugaku Zasshi. 128 (6): 981–7.
- ↑ Fisher MB, Henne KR, Boer J (2006). "The complexities inherent in attempts to decrease drug clearance by blocking sites of CYP-mediated metabolism". Current Opinion in Drug Discovery & Development. 9 (1): 101–9. PMID 16445122.
- ↑ Harvey, J. A. (1978). "Neurotoxic Action of Halogenated Amphetamines". Annals of the New York Academy of Sciences. 305: 289–304. doi:10.1111/j.1749-6632.1978.tb31530.x. PMID 81648.
- ↑ Fuller, R. W.; Baker, J. C.; Perry, K. W.; Molloy, B. B. (1975). "Comparison of 4-chloro-, 4-bromo- and 4-fluoroamphetamine in rats: Drug levels in brain and effects on brain serotonin metabolism". Neuropharmacology. 14 (10): 739–746. doi:10.1016/0028-3908(75)90099-4. PMID 1196472.
- ↑ Nichols, DE; Johnson, MP; Oberlender, R (1991). "5-Iodo-2-aminoindan, a nonneurotoxic analogue of p-iodoamphetamine". Pharmacology, Biochemistry, and Behavior. 38 (1): 135–9. doi:10.1016/0091-3057(91)90601-W. PMID 1826785.
- ↑ Rothman, R. B.; Blough, BE; Woolverton, WL; Anderson, KG; Negus, SS; Mello, NK; Roth, BL; Baumann, MH (2005). "Development of a Rationally Designed, Low Abuse Potential, Biogenic Amine Releaser That Suppresses Cocaine Self-Administration". Journal of Pharmacology and Experimental Therapeutics. 313 (3): 1361–1369. doi:10.1124/jpet.104.082503. PMID 15761112.
- ↑ Blanckaert, P.; van Amsterdam, Jgc; Brunt, Tm; van den Berg, Jdj; Van Durme, F.; Maudens, K.; van Bussel, Jch (2013-09-01). "4-Methyl-amphetamine: a health threat for recreational amphetamine users". Journal of Psychopharmacology (Oxford, England). 27 (9): 817–822. doi:10.1177/0269881113487950. ISSN 1461-7285. PMID 23784740.
- ↑ Huang, X.; Marona-Lewicka, D.; Nichols, D. E. (1992). "P-methylthioamphetamine is a potent new non-neurotoxic serotonin-releasing agent". European Journal of Pharmacology. 229 (1): 31–38. doi:10.1016/0014-2999(92)90282-9. PMID 1473561.
- ↑ Li, Q; Murakami, I; Stall, S; Levy, AD; Brownfield, MS; Nichols, DE; Van De Kar, LD (1996). "Neuroendocrine pharmacology of three serotonin releasers: 1-(1,3-benzodioxol-5-yl)-2-(methylamino)butane (MBDB), 5-methoxy-6-methyl-2-aminoindan (MMAi) and p-methylthioamphetamine (MTA)". The Journal of Pharmacology and Experimental Therapeutics. 279 (3): 1261–7. PMID 8968349.
- ↑ Murphy, J; Flynn, JJ; Cannon, DM; Guiry, PJ; McCormack, P; Baird, AW; McBean, GJ; Keenan, AK (2002). "In vitro neuronal and vascular responses to 5-hydroxytryptamine: modulation by 4-methylthioamphetamine, 4-methylthiomethamphetamine and 3,4-methylenedioxymethamphetamine". European Journal of Pharmacology. 444 (1–2): 61–7. doi:10.1016/S0014-2999(02)01586-8. PMID 12191583.
- ↑ E. Costa; S. Garattini (1970). Amphetamines and Related Compounds. New York: Raven Press. p. 28.
- ↑ Lapoint, Jeff; Dargan, Paul I.; Hoffman, Robert S. (2013). "7 – Synthetic Amphetamine Derivatives". Novel Psychoactive Substances. Academic Press.
- ↑ "关于印发《非药用类麻醉药品和精神药品列管办法》的通知" (in Chinese). China Food and Drug Administration. 27 September 2015. Retrieved 1 October 2015.
- ↑ "Látky, o které byl doplněn seznam č. 4 psychotropních látek (příloha č. 4 k nařízení vlády č. 463/2013 Sb.)" (PDF) (in Czech). Ministerstvo zdravotnictví.