Grey matter
| Grey matter | |
|---|---|
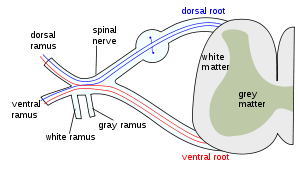 The formation of the spinal nerve from the dorsal and ventral roots. (Grey matter labeled at center right.) | |
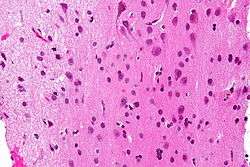 Micrograph showing grey matter, with the characteristic neuronal cell bodies (dark shade of pink), and white matter with its characteristic fine meshwork-like appearance (left of image - lighter shade of pink). HPS stain. | |
| Details | |
| Identifiers | |
| Latin | Substantia grisea |
| TA | A14.1.00.002 |
| FMA | 67242 |
Grey matter (or gray matter) is a major component of the central nervous system, consisting of neuronal cell bodies, neuropil (dendrites and myelinated as well as unmyelinated axons), glial cells (astroglia and oligodendrocytes), synapses, and capillaries. Grey matter is distinguished from white matter, in that it contains numerous cell bodies and relatively few myelinated axons, while white matter contains relatively very few cell bodies and is composed chiefly of long-range myelinated axon tracts.[1] The colour difference arises mainly from the whiteness of myelin. In living tissue, grey matter actually has a very light grey colour with yellowish or pinkish hues, which come from capillary blood vessels and neuronal cell bodies.[2]
Structure
Grey matter refers to unmyelinated neurons and other cells of the central nervous system. It is present in the brain, brainstem and cerebellum, and present throughout the spinal cord.
Grey matter is distributed at the surface of the cerebral hemispheres (cerebral cortex) and of the cerebellum (cerebellar cortex), as well as in the depths of the cerebrum (thalamus; hypothalamus; subthalamus, basal ganglia – putamen, globus pallidus, nucleus accumbens; septal nuclei), cerebellar (deep cerebellar nuclei – dentate nucleus, globose nucleus, emboliform nucleus, fastigial nucleus), brainstem (substantia nigra, red nucleus, olivary nuclei, cranial nerve nuclei).
Grey matter in the spinal cord is known as the grey column which travels down the spinal cord distributed in three grey columns that are presented in an "H" shape. The forward-facing column is the anterior grey column, the rear-facing one is the posterior grey column and the interlinking one is the lateral grey column. The grey matter on the left and right side is connected by the gray commissure. The grey matter in the spinal cord consists of interneurons, as well as cell bodies.
-
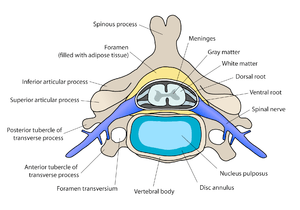
Diagram of a spinal vertebra. The grey matter is in the central part of the spinal cord.
-
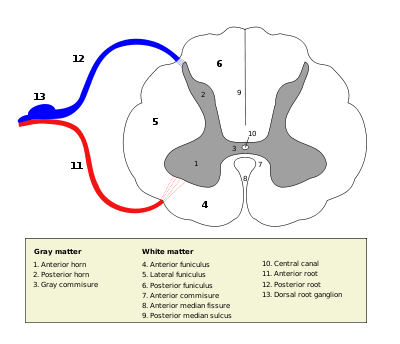
Cross-section of spinal cord with grey matter labelled.
Grey matter undergoes development and growth throughout childhood and adolescence.[3]
Function
Grey matter contains most of the brain's neuronal cell bodies. The grey matter includes regions of the brain involved in muscle control, and sensory perception such as seeing and hearing, memory, emotions, speech, decision making, and self-control.[4]
The grey matter in the spinal cord is split into three grey columns:
- The anterior grey column contains motor neurons. These synapse with interneurons and the axons of cells that have travelled down the pyramidal tract. These cells are responsible for the movement of muscles.
- The posterior grey column contains the points where sensory neurons synapse. These receives sensory information from the body, including fine touch, proprioception, and vibration. This information is sent from receptors of the skin, bones, and joints through sensory neurons whose cell bodies lie in the dorsal root ganglion. This information is then transmitted in axons up the spinal cord in spinal tracts, including the dorsal column-medial lemniscus tract and the spinothalamic tract.
- The lateral grey column is the third column of the spinal cord.
The grey matter of the spinal cord can be divided into different layers, called Rexed laminae. These describe, in general, the purpose of the cells within the grey matter of the spinal cord at a particular location.
-

Interneurons present in the grey matter of the spinal cord
-
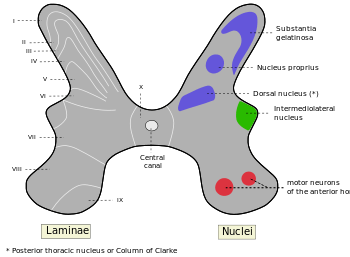
Rexed laminae groups the grey matter in the spinal cord according to its function.
Clinical significance
Research
Volume and cognition in elderly people
Significant positive correlations have been found between grey matter volume in elderly persons and measures of semantic and short-term memory. No significant correlations with white matter volume were found. These results suggest that individual variability in specific cognitive functions that are relatively well preserved with aging is accounted for by the variability of grey matter volume in healthy elderly subjects.[5]
Volume associated with bipolar disorder, and substance misuse
Some structural differences in grey matter may be associated with psychiatric disorders. There was no difference in whole-brain grey matter volume between patients with bipolar I disorder and healthy controls. Subjects with bipolar I disorder had smaller volumes in the left inferior parietal lobule, right superior temporal gyrus, right middle frontal gyrus, and left caudate. Only the volume of the right middle frontal gyrus was correlated with duration of illness and the number of episodes in patients.[6]
It has been found that adolescents suffering from bipolar disorder are more likely to develop substance use disorders if they have lower gray matter volume.[7]
Volume associated with smoking
Older smokers lose grey matter and cognitive function at a greater rate than non-smokers. Chronic smokers who quit during the study lost fewer brain cells and retained better intellectual function than those who continued to smoke.[8]
Researchers discovered less grey matter in brains of men who view pornography in large amounts compared with those who do not. Yet a direct influence of pornographic material on shrinking size of grey matter could not have been made. Further studies are urged.[9][10]
Volume associated with alcohol use
High alcohol consumption has been correlated with significant reductions in grey matter volume.[11][12]
Volume associated with poverty
Numerous reports have shown that children in lower-income families do worse on average on IQ and standardized tests compared to children from wealthier families. Later research has investigated the associations between poverty and neural development. This has shown an association between poverty and lower volume and surface area of grey matter in children growing up in poverty.[13] A U.S. study found that at birth there was no significant difference in total grey matter of newborn babies, but at the age of 2 a significant difference in grey matter was found, and by the age of 4 this difference was further pronounced.[14] Test scores have been linked to atypical structural development in the brain, with children living in poverty having a regional grey matter that was 3-4 percentage points below what was deemed the developmental-norm.[13] Similarly, a 2015 study conducted in the USA compared cortical grey matter of 8th graders in Massachusetts. Children from lower-income families not only scored lower in maths and English in standardised tests, but MRI showed that these children statistically had significantly lower grey matter volume and cortical thickness of the bilateral temporal and occipital lobes, compared to children from higher income families.[15] No significant differences were found in white matter volumes. Regional differences are not consistent from study to study. For instance, previous research examined prefrontal cortical thickness in healthy children – a region deemed essential to executive function, which is in turn associated with academic success, and has a long developmental trajectory, which may be susceptible to environmental factors. In a sub-sample of cases from a larger pool of 433 subjects, MRI scans showed the right anterior cingulate gyrus and the left frontal gyrus were significantly correlated with poverty.[16]
Volume associated with subjective happiness score
A positive relationship has been found between the volume of grey matter in the right precuneus and the subject's subjective happiness score.[17]
Volume associated with mindfulness
A 6-week mindfulness based intervention was found to correlate with a significant grey matter increase within the precuneus.[18]
History
Etymology
In the current edition[19] of the official Latin nomenclature, Terminologia Anatomica, substantia grisea is used for English grey matter. The adjective grisea for grey is however not attested in classical Latin.[20] The adjective grisea is derived from the French word for grey, gris.[20] Alternative designations like substantia cana [21] and substantia cinerea [22] are being used alternatively. The adjective cana, attested in classical Latin,[23] can mean grey,[20] or greyish white.[24] The classical Latin cinerea means ash-coloured.[23]
Additional images
-
Human brain right dissected lateral view
-
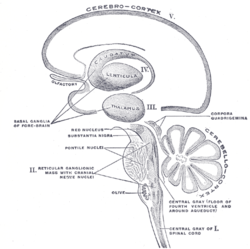
Schematic representation of the chief ganglionic categories (I to V).
See also
References
- ↑ Purves, Dale; George J. Augustine; David Fitzpatrick; William C. Hall; Anthony-Samuel LaMantia; James O. McNamara; Leonard E. White (2008). Neuroscience (4th ed.). Sinauer Associates. pp. 15–16. ISBN 978-0-87893-697-7.
- ↑ Kolb & Whishaw: Fundamentals of Human Neuropsychology (2003) page 49
- ↑ Sowell ER, Thompson PM, Tessner KD, Toga AW (15 November 2001). "Mapping Continued Brain Growth and Gray Matter Density Reduction in Dorsal Frontal Cortex: Inverse Relationships during Postadolescent Brain Maturation". The Journal of Neuroscience. PMID 11698594.
- ↑ Miller, A. K. H.; Alston, Corsellis (28 June 2008). "VARIATION WITH AGE IN THE VOLUMES OF GREY AND WHITE MATTER IN THE CEREBRAL HEMISPHERES OF MAN: MEASUREMENTS WITH AN IMAGE ANALYSER". Neuropathology and Applied Neurobiology. 6 (2): 119–132. doi:10.1111/j.1365-2990.1980.tb00283.x. PMID 7374914.
- ↑ Taki, Y; Kinomura, S; Sato, K; Goto, R; Wu, K; Kawashima, R; Fukuda, H (March 2011). "Correlation between gray/white matter volume and cognition in healthy elderly people.". Brain and cognition. 75 (2): 170–176. doi:10.1016/j.bandc.2010.11.008. PMID 21131121. (subscription required)
- ↑ Li, M; Cui, L; Deng, W; Ma, X; Huang, C; Jiang, L; Wang, Y; Collier, DA; Gong, Q; Li, T (February 28, 2011). "Voxel-based morphometric analysis on the volume of gray matter in bipolar I disorder". Psychiatry Res. 191 (2): 92–97. doi:10.1016/j.pscychresns.2010.09.006. PMID 21236649. (subscription required)
- ↑ http://medicine.yale.edu/news/article.aspx?id=13842
- ↑ "Smoking causes brain cell loss and cognitive decline". The University of Western Australia. 9 February 2011. Retrieved 2011-04-21.
- ↑ Tom Payne (30 May 2014). "Why watching too much porn could be bad for the brain". independent.co.uk.
- ↑ "Does porn affect the brain? Scientists urge more study", Retrieved December 4, 2015.
- ↑ http://www.sciencedirect.com/science/article/pii/S0149763415302451
- ↑ http://www.drugandalcoholdependence.com/article/S0376-8716(15)00266-5/abstract?rss=yes
- 1 2 Hair, N. L., Hanson, J. L., Wolfe, B. L., & Pollak, S. D. (2015). Association of child poverty, brain development, and academic achievement. JAMA pediatrics, 169(9), 822-829
- ↑ Hanson, J. L., Hair, N., Shen, D. G., Shi, F., Gilmore, J. H., Wolfe, B. L., & Pollak, S. D. (2013). Family poverty affects the rate of human infant brain growth.
- ↑ Mackey, A. P., Finn, A. S., Leonard, J. A., Jacoby-Senghor, D. S., West, M. R., Gabrieli, C. F., & Gabrieli, J. D. (2015). Neuroanatomical correlates of the income achievement gap. Psychological science, 0956797615572233.
- ↑ Lawson, G. M., Duda, J. T., Avants, B. B., Wu, J., & Farah, M. J. (2013). Associations between children's socioeconomic status and prefrontal cortical thickness. Developmental science, 16(5), 641-652.
- ↑ http://www.nature.com/articles/srep16891
- ↑ Kurth F, Luders E, Wu B, Black DS (2014). "Brain Gray Matter Changes Associated with Mindfulness Meditation in Older Adults: An Exploratory Pilot Study using Voxel-based Morphometry". Neuro. 1: 23–26. doi:10.17140/NOJ-1-106. PMC 4306280
 . PMID 25632405.
. PMID 25632405. - ↑ Federative Committee on Anatomical Terminology (FCAT) (1998). Terminologia Anatomica. Stuttgart: Thieme
- 1 2 3 Triepel, H. (1910). Die anatomischen Namen. Ihre Ableitung und Aussprache. Mit einem Anhang: Biographische Notizen.(Dritte Auflage). Wiesbaden: Verlag J.F. Bergmann.
- ↑ Triepel, H. (1910). Nomina Anatomica. Mit Unterstützung von Fachphilologen. Wiesbaden: Verlag J.F. Bergmann.
- ↑ Schreger, C.H.Th.(1805). Synonymia anatomica. Synonymik der anatomischen Nomenclatur. Fürth: im Bureau für Literatur.
- 1 2 Lewis, C.T. & Short, C. (1879). A Latin dictionary founded on Andrews' edition of Freund's Latin dictionary. Oxford: Clarendon Press.
- ↑ Stearn, W.T. (1983). Botanical Latin. History, grammar, syntax, terminology and vocabulary. (3rd edition). Newton Abbot London: David Charles.