Hampson–Linde cycle
| Thermodynamics | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
The classical Carnot heat engine | ||||||||||||
|
Branches |
||||||||||||
|
||||||||||||
| Book:Thermodynamics | ||||||||||||
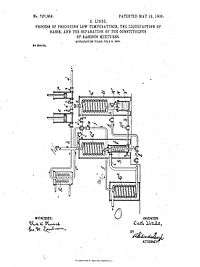
The Hampson–Linde cycle is used in the liquefaction of gases, especially for air separation. William Hampson and Carl von Linde independently filed for patent of the cycle in 1895.[1]
Hampson-Linde systems introduced regenerative cooling, a positive-feedback cooling system. The heat exchanger arrangement permits an absolute temperature difference (e.g. 0.27 °C/atm J-T cooling for air) to go beyond a single stage of cooling, and reach the low temperatures required to liquefy "fixed" gasses.
The Hampson-Linde cycle differs from the Siemens cycle only in the expansion step. Where the Siemens cycle has the gas do external work to reduce its temperature, the Hampson-Linde cycle relies solely on the Joule-Thomson effect. This has the advantage that the cold side needs no moving parts.[1]
The cycle

- Heated—by compressing the gas—adding external energy into the gas, to give it what is needed for running through the cycle
- Cooled—by immersing the gas in a cooler environment, losing some of its heat (and energy),
- Cooled through heat exchanger with returning gas from next (and last) stage,
- Cooled further by passing the gas through a Joule-Thomson orifice, removing heat, but conserving energy which is now potential energy rather than kinetic energy.
- The gas which is now at its coolest in the current cycle, is recycled and sent back to be -
- Heated—when participating as the coolant for stage 3, and then
- Sent back to stage one, to start the next cycle, and be slightly reheated by compression.
In each cycle the net cooling is more than the heat added at the beginning of the cycle. As the gas passes more cycles and becomes cooler, reaching lower temperatures at the expanding cylinder becomes more difficult.
Further reading
- Timmerhaus, Klaus D.; Reed, Richard Palmer (2007). Cryogenic Engineering: Fifty Years of Progress. p. 8. ISBN 978-0-387-46896-9.
- Almqvist, Ebbe (2003). History of industrial gases. p. 160. ISBN 0-306-47277-5.
References
- 1 2 "Technical information". Kryolab, Lund University. Retrieved 26 January 2013.
- Maytal, B. -Z. (2006). "Maximizing production rates of the Linde–Hampson machine". Cryogenics. 46: 49–85. Bibcode:2006Cryo...46...49M. doi:10.1016/j.cryogenics.2005.11.004.
