Kinetochore
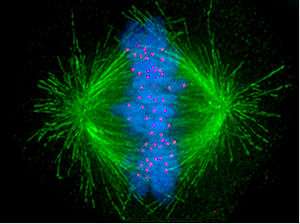
The kinetochore (/kᵻˈnɛtəkɔər/, /-ˈniːtəkɔər/) is a protein structure on chromatids where the spindle fibers attach during cell division to pull sister chromatids apart. Their proteins help to hold the sister chromatids together and also play a role in chromosome editing.[1]
The kinetochore forms in eukaryotes, assembles on the centromere and links the chromosome to microtubule polymers from the mitotic spindle during mitosis and meiosis.
Monocentric organisms, including vertebrates, fungi, and most plants, have a single centromeric region on each chromosome which assembles one kinetochore. Holocentric organisms, such as nematodes and some plants, assemble a kinetochore along the entire length of a chromosome.[2]
The kinetochore contains two regions:
- an inner kinetochore, which is tightly associated with the centromere DNA, assembled in a specialized form of chromatin persistent throughout the cell cycle;
- an outer kinetochore, which interacts with microtubules; the outer kinetochore is a very dynamic structure, with many identical components, which are assembled and functional only during cell division.
Kinetochores start, control, and supervise the striking movements of chromosomes during cell division. During mitosis, which occurs after chromosomes are duplicated during S phase, two sister chromatids are held together, each with its own kinetochore, which face in opposing directions and attach to opposite poles of the mitotic spindle. Following the transition from metaphase to anaphase, the sister chromatids separate from each other, and the individual kinetochores on each chromatid drive their movement to the spindle poles that will define the two new daughter cells. Thus, the kinetochore is essential for the chromosome segregation that is classically associated with mitosis and meiosis.
Even the simplest kinetochores consist of more than 19 different proteins. Many of these proteins are conserved between eukaryotic species, including a specialized histone H3 variant (called CENP-A or CenH3) which helps the kinetochore associate with DNA. Other proteins in the kinetochore attach it to the microtubules (MTs) of the mitotic spindle. There are also motor proteins, including both dynein and kinesin, which generate forces that move chromosomes during mitosis. Other proteins, such as Mad2, monitor the microtubule attachment as well as the tension between sister kinetochores and activate the spindle checkpoint to arrest the cell cycle when either of these is absent.[3]
In summary, kinetochore functions include anchoring of chromosomes to MTs in the spindle, verification of anchoring, activation of the spindle checkpoint and participation in force generation to propel chromosome movement during cell division.[4]
On the other hand, MTs are metastable polymers made of α- and β-tubulin, alternating between growing and shrinking phases, a phenomenon known as dynamic instability.[5] MTs are highly dynamic structures, whose behavior is integrated with kinetochore function to control chromosome movement and segregation.
Structure in animal cells
The kinetochore is composed of several layers, observed initially by conventional fixation and staining methods of electron microscopy,[6][7] (reviewed by C. Rieder in 1982[8]) and more recently by rapid freezing and substitution.[9]
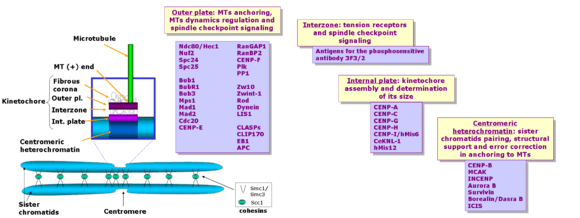
The deepest layer in the kinetochore is the inner plate, which is organized on a chromatin structure containing nucleosomes presenting a specialized histone (named CENP-A, which substitutes histone H3 in this region), auxiliary proteins and DNA. DNA organization in the centromere (satellite DNA) is one of the least known aspects in vertebrate kinetochores. The inner plate appears like a discrete heterochromatin domain throughout the cell cycle.
Outside the inner plate we find the outer plate, composed mostly by proteins. This structure is assembled in the surface of the chromosomes when the nuclear envelope breaks down.[6] The outer plate in vertebrate kinetochores contains about 20 anchoring sites for MTs (+) ends (named kMTs, after kinetochore MTs), whereas a kinetochore's outer plate in yeast (Saccharomyces cerevisiae) contains only one anchoring site.
The outermost domain in the kinetochore forms a fibrous corona, which can be visualized by conventional microscopy, yet only in absence of MTs. This corona is formed by a dynamic network of resident and temporary proteins implicated in the spindle checkpoint, in MTs anchoring and in the regulation of chromosome behavior.
During mitosis, each sister chromatid forming the complete chromosome has its own kinetochore. Distinct sister kinetochores can be observed at first at the end of G2 phase in cultured mammalian cells.[10] These early kinetochores show a mature laminar structure before the nuclear envelope breaks down (reviewed by Pluta et al. in 1995[11]). The molecular pathway for kinetochore assembly in higher eukaryotes has been studied using gene knockouts in mice and in cultured chicken cells, as well as using RNA interference (RNAi) in C. elegans, Drosophila and human cells. Yet no simple linear route can describe the data obtained so far.
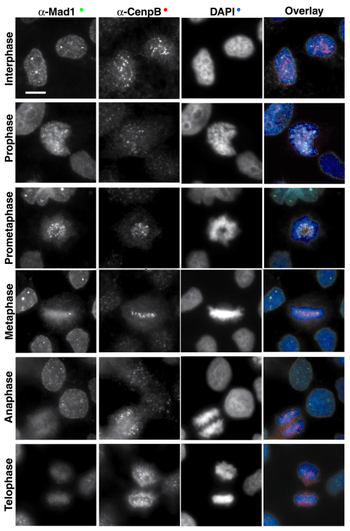
The first protein to be assembled on the kinetochore is CENP-A (Cse4 in Saccharomyces cerevisiae). This protein is a specialized isoform of histone H3.[12] CENP-A is required for incorporation of the inner kinetochore proteins CENP-C, CENP-H and CENP-I/MIS6.[13][14][15][16][17] The relation of these proteins in the CENP-A dependent pathway is not completely defined. For instance, CENP-C localization requires CENP-H in chicken cells, but it is independent of CENP-I/MIS6 in human cells. In C. elegans and metazoa, the incorporation of many proteins in the outer kinetochore depends ultimately on CENP-A.
Kinetochore proteins can be grouped according to their concentration at kinetochores during mitosis: some proteins remain bound throughout cell division, whereas some others change in concentration; furthermore, they can be recycled in their binding site on kinetochores either slowly (they are rather stable) or rapidly (dynamic).
- Proteins whose levels remain stable from prophase until late anaphase include constitutive components of the inner plate and the stable components of the outer kinetocore, such as the Ndc80 complex,[18][19] KNL/KBP proteins (kinetochore-null/KNL-binding protein),[20] MIS proteins[20] and CENP-F.[21][22] Together with the constitutive components, these proteins seem to organize the nuclear core of the inner and outer structures in the kinetochore.
- The dynamic components that vary in concentration on kinetochores during mitosis include the molecular motors CENP-E and dynein (as well as their target components ZW10 and ROD), and the spindle checkpoint proteins (such as Mad1, Mad2, BubR1 and Cdc20). These proteins assemble on the kinetochore in high concentration in absence of microtubules; however, the higher the number of MTs anchored to the kinetochore, the lower the concentration of these proteins.[23] At metaphase, CENP-E, Bub3 and Bub1 levels disminish about 3 to 4x as compared to free kinetochores, whereas dynein/dynactin, Mad1, Mad2 and BubR1 levels are reduced >10-100x.[23][24][25][26]
- Whereas the spindle checkpoint protein levels present in the outer plate diminish as MTs anchor,[26] other components such as EB1, APC and proteins in the Ran pathway (RanGap1 and RanBP2) associate to kinetochores only when MTs are anchored.[27][28][29][30] This may belong to a mechanism in the kinetochore to recognize the MTs plus-end (+), ensuring their proper anchoring and regulating their dynamic behavior as they remain anchored.
A 2010 study uses a complex method (termed multiclassifier combinatorial proteomics or MCCP) to analyze the proteomic composition of vertebrate chromosomes, including kinetochores.[31] Although this study does not include a biochemical enrichment for kinetochores, obtained data include all the centromeric subcomplexes, with peptides from all 125 known centromeric proteins. According to this study, there are still about one hundred unknown kinetochore proteins, doubling the known structure during mitosis, which confirms the kinetochore as one of the most complex cellular substructures. Consistently, a comprehensive literature survey indicated that there had been at least 196 human proteins already experimentally shown to be localized at kinetochores.[32]
Function
The number of MTs attached to one kinetochore is variable: in Saccharomyces cerevisiae only one MT binds each kinetochore, whereas in mammals there can be among 15-35 MTs bound to each kinetochore.[33] However, not all the MTs in the spindle attach to one kinetochore. There are MTs that extend from one centrosome to the other (and they are responsible for spindle length) and some shorter ones are interdigitated between the long MTs. Professor B. Nicklas (Duke University), showed that, if one breaks down the MT-kinetochore attachment using a laser beam, chromatids can no longer move, leading to an abnormal chromosome distribution.[34] These experiments also showed that kinetochores have polarity, and that kinetochore attachment to MTs emanating from one or the other centrosome will depend on its orientation. This specificity guarantees that only one chromatid will move to each spindle side, thus ensuring the correct distribution of the genetic material. Thus, one of the basic functions of the kinetochore is the MT attachment to the spindle, which is essential to correctly segregate sister chromatids. If anchoring is incorrect, errors may ensue, generating aneuploidy, with catastrophic consequences for the cell. To prevent this from happening, there are mechanisms of error detection and correction (as the spindle assembly checkpoint), whose components reside also on the kinetochores.The movement of one chromatid towards the centrosome is produced primarily by MT depolymerization in the binding site with the kinetochore. These movements require also force generation, involving molecular motors likewise located on the kinetochores.
Chromosome anchoring to MTs in the mitotic spindle
Capturing MTs
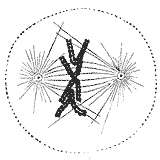
During the synthesis phase (S phase) in the cell cycle, the centrosome starts to duplicate. Just at the beginning of mitosis, both centrioles in each centrosome reach their maximal length, centrosomes recruit additional material and their nucleation capacity for microtubules increases. As mitosis progresses, both centrosomes separate to establish the mitotic spindle.[35] In this way, the spindle in a mitotic cell has two poles emanating microtubules. Microtubules are long proteic filaments with asymmetric extremes, a "minus"(-) end relatively stable next to the centrosome, and a "plus"(+) end enduring alternate phases of growing-shrinking, exploring the center of the cell. During this searching process, a microtubule may encounter and capture a chromosome through the kinetochore.[36][37] Microtubules that find and attach a kinetochore become stabilized, whereas those microtubules remaining free are rapidly depolymerized.[38] As chromosomes have two kinetochores associated back-to-back (one on each sister chromatid), when one of them becomes attached to the microtubules generated by one of the cellular poles, the kinetochore on the sister chromatid becomes exposed to the opposed pole; for this reason, most of the times the second kinetochore becomes attached to the microtubules emanating from the opposing pole,[39] in such a way that chromosomes are now bi-oriented, one fundamental configuration (also termed amphitelic) to ensure the correct segregation of both chromatids when the cell will divide.[40][41]
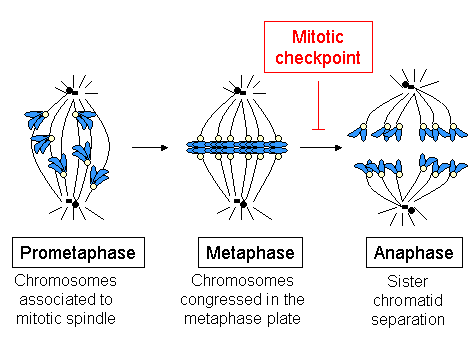
When just one microtubule is anchored to one kinetochore, it starts a rapid movement of the associated chromosome towards the pole generating that microtubule. This movement is probably mediated by the motor activity towards the "minus" (-) of the motor protein cytoplasmic dynein,[42][43] which is very concentrated in the kinetochores not anchored to MTs.[44] The movement towards the pole is slowed down as far as kinetochores acquire kMTs (MTs anchored to kinetochores) and the movement becomes directed by changes in kMTs length. Dynein is released from kinetochores as they acquire kMTs[23] and, in cultured mammalian cells, it is required for the spindle checkpoint inactivation, but not for chromosome congression in the spindle equator, kMTs acquisition or anaphase A during chromosome segregation.[45] In higher plants or in yeast there is no evidence of dynein, but other kinesins towards the (-) end might compensate for the lack of dynein.
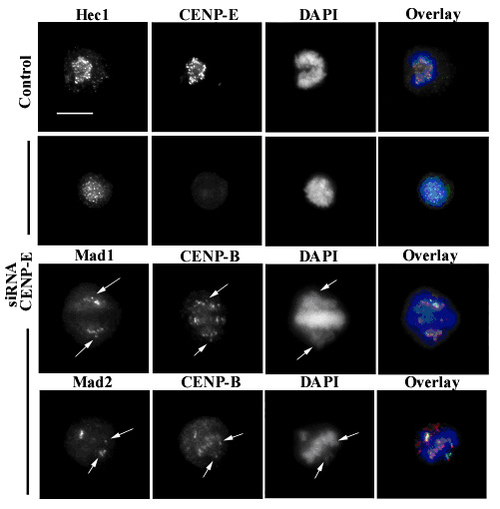
Another motor protein implicated in the initial capture of MTs is CENP-E; this is a high molecular weight kinesin associated with the fibrous corona at mammalian kinetochores from prometaphase until anaphase.[46] In cells with low levels of CENP-E, chromosomes lack this protein at their kinetochores, which quite often are defective in their ability to congress at the metaphase plate. In this case, some chromosomes may remain chronically mono-oriented (anchored to only one pole), although most chromosomes may congress correctly at the metaphase plate.[47]
Generally it is widely accepted that the kMTs fiber (the bundle of microtubules bound to the kinetochore) is originated by the capture of MTs polymerized at the centrosomes and spindle poles in mammalian cultured cells.[36] However, MTs directly polymerized at kinetochores might also contribute significantly.[48] The manner in which the centromeric region or kinetochore initiates the formation of kMTs and the frequency at which this happens are important questions, because this mechanism may contribute not only to the initial formation of kMTs, but also to the way in which kinetochores correct defective anchoring of MTs and regulate the movement along kMTs.
Role of Ndc80 complex
MTs associated to kinetochores present special features: compared to free MTs, kMTs are much more resistant to cold-induced depolymerization, high hydrostatic pressures or calcium exposure.[49] Furthermore, kMTs are recycled much more slowly than astral MTs and spindle MTs with free (+) ends, and if kMTs are released from kinetochores using a laser beam, they rapidly depolymerize.[34]
When it was clear that neither dynein nor CENP-E is essential for kMTs formation, other molecules should be responsible for kMTs stabilitation. Pioneer genetic work in yeast revealed the relevance of the Ndc80 complex in kMTs anchoring.[18][50][51][52] In Saccharomyces cerevisiae, the Ndc80 complex has four components: Ndc80p, Nuf2p, Spc24p and Spc25p. Mutants lacking any of the components of this complex show loss of the kinetochore-microtubule connection, although kinetochore structure is not completely lost.[18][50] Yet mutants in which kinetochore structure is lost (for instance Ndc10 mutants in yeast[53]) are deficient both in the connection to microtubules and in the ability to activate the spindle checkpoint, probably because kinetochores work as a platform in which the components of the response are assembled.
The Ndc80 complex is highly conserved and it has been identified in S. pombe, C. elegans, Xenopus, chicken and humans.[18][19][50][54][55][56][57] Studies on Hec1 (highly expressed in cancer cells 1), the human homolog of Ndc80p, show that it is important for correct chromosome congression and mitotic progression, and that it interacts with components of the cohesin and condensin complexes.[58]
Different laboratories have shown that the Ndc80 complex is essential for stabilization of the kinetochore-microtubule anchoring, required to support the centromeric tension implicated in the establishment of the correct chromosome congression in high eukaryotes.[19][55][56][57] Cells with impaired function of Ndc80 (using RNAi, gene knockout, or antibody microinjection) have abnormally long spindles, lack of tension between sister kinetochores, chromosomes unable to congregate at the metaphase plate and few or any associated kMTs.
There is a variety of strong support for the ability of the Ndc80 complex to directly associate with microtubules and form the core conserved component of the kinetochore-microtubule interface.[59] However, formation of robust kinetochore-microtubule interactions may also require the function of additional proteins. In yeast, this connection requires the presence of the complex Dam1-DASH-DDD. Some members of this complex bind directly to MTs, whereas some others bind to the Ndc80 complex.[51][52][60] This means that the complex Dam1-DASH-DDD might be an essential adapter between kinetochores and microtubules. However, in animals an equivalent complex has not been identified, and this question remains under intense investigation.
Verification of kinetochore-MT anchoring
When a cell enters in mitosis, it duplicates all the genetic information stored in the chromosomes, in the process termed DNA replication. At the end of this process, each chromosome includes two sister chromatids, which are two complete and identical DNA molecules. Both chromatids remain associated by cohesin complexes until anaphase, when chromosome segregation occurs. If chromosome segregation happens correctly, each daughter cell receives a complete set of chromatids, and for this to happen each sister chromatid has to anchor (through the corresponding kinetochore) to MTs generated in opposed poles of the mitotic spindle. This configuration is termed amphitelic or bi-orientation.
However, during the anchoring process some incorrect configurations may also appear:[61]
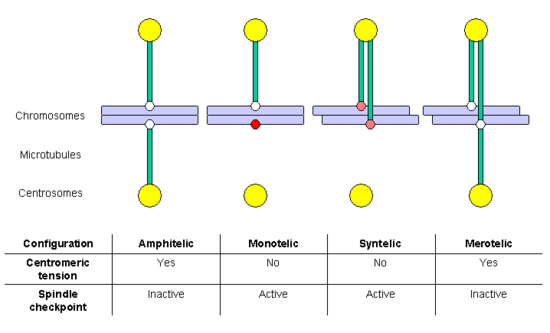
- monotelic: only one of the chromatids is anchored to MTs, the second kinetochore is not anchored; in this situation, there is no centromeric tension, and the spindle checkpoint is activated, delaying entry in anaphase and allowing time for the cell to correct the error. If it is not corrected, the unanchored chromatid might randomly end in any of the two daughter cells, generating aneuploidy: one daughter cell would have chromosomes in excess and the other would lack some chromosomes.
- syntelic: both chromatids are anchored to MTs emanating from the same pole; this situation does not generate centromeric tension either, and the spindle checkpoint will be activated. If it is not corrected, both chromatids will end in the same daughter cell, generating aneuploidy.
- merotelic: at least one chromatid is anchored simultaneously to MTs emanating from both poles. This situation generates centromeric tension, and for this reason the spindle checkpoint is not activated. If it is not corrected, the chromatid bound to both poles will remain as a lagging chromosome at anaphase, and finally will be broken in two fragments, distributed between the daughter cells, generating aneuploidy.
Both the monotelic and the syntelic configurations fail to generate centromeric tension and are detected by the spindle checkpoint. In contrast, the merotelic configuration is not detected by this control mechanism. However, most of these errors are detected and corrected before the cell enters in anaphase.[61] A key factor in the correction of these anchoring errors is the chromosomal passenger complex, which includes the kinase protein Aurora B, its target and activating subunit INCENP and two other subunits, Survivin and Borealin/Dasra B (reviewed by Adams and collaborators in 2001[62]). Cells in which the function of this complex has been abolished by dominant negative mutants, RNAi, antibody microinjection or using selective drugs, accumulate errors in chromosome anchoring. Many studies have shown that Aurora B is required to destabilize incorrect anchoring kinetochore-MT, favoring the generation of amphitelic connections. Aurora B homolog in yeast (Ipl1p) phosphorilates some kinetochore proteins, such as the constitutive protein Ndc10p and members of the Ndc80 and Dam1-DASH-DDD complexes.[63] Phosphorilation of Ndc80 complex components produces destabilization of kMTs anchoring. It has been proposed that Aurora B localization is important for its function: as it is located in the inner region of the kinetochore (in the centromeric heterochromatin), when the centromeric tension is established sister kinetochores separate, and Aurora B cannot reach its substrates, so that kMTs are stabilized. It is interesting to note that Aurora B is frequently overexpressed in several cancer types, and it is currently a target for the development of anticancer drugs.[64]
Spindle checkpoint activation
The spindle checkpoint or SAC (for spindle assembly checkpoint), also known as mitotic checkpoint, is a cellular mechanism responsible for detection of:
- correct assembly of the mitotic spindle
- attachment of all chromosomes to the mitotic spindle in a bipolar manner
- congression of all chromosomes at the metaphase plate.
When just one chromosome (for any reason) remains lagging during congression, the spindle checkpoint machinery generates a delay in cell cycle progression: the cell is arrested, allowing time for repair mechanisms to solve the detected problem. After some time, if the problem has not been solved, the cell will be targeted for apoptosis (programmed cell death), a safety mechanism to avoid the generation of aneuploidy, a situation which generally has dramatic consequences for the organism.
Whereas structural centromeric proteins (such as CENP-B), remain stably localized throughout mitosis (including telophase), the spindle checkpoint components are assembled on the kinetochore in high concentrations in absence of MTs, and their concentration decreases as the number of MTs attached to the kinetochore increases.[23]
At metaphase, CENP-E, Bub3 and Bub1 levels decreases 3 to 4 fold as compared to the levels at unattached kinetochores, whereas the levels of dynein/dynactin, Mad1, Mad2 and BubR1 decrease >10-100 fold.[23][24][25][26] Thus at metaphase, when all chromosomes are aligned at the metaphase plate, all checkpoint proteins are released from the kinetochore. The disappearance of the checkpoint proteins out of the kinetochores indicates the moment when the chromosome has reached the metaphase plate and is under bipolar tension. At this moment, the checkpoint proteins that bind to and inhibit Cdc20 (Mad1-Mad2 and BubR1), release Cdc20, which binds and activates APC/CCdc20, and this complex triggers sister chromatids separation and consequently anaphase entry.
Several studies indicate that the Ndc80 complex participates in the regulation of the stable association of Mad1-Mad2 and dynein with kinetochores.[19][56][57] Yet the kinetochore associated proteins CENP-A, CENP-C, CENP-E, CENP-H and BubR1 are independent of Ndc80/Hec1. The prolonged arrest in prometaphase observed in cells with low levels of Ndc80/Hec1 depends on Mad2, although these cells show low levels of Mad1, Mad2 and dynein on kinetochores (<10-15% in relation to unattached kinetochores). However, if both Ndc80/Hec1 and Nuf2 levels are reduced, Mad1 and Mad2 completely disappear from the kinetochores and the spindle checkpoint is inactivated.[65]
Shugoshin (Sgo1, MEI-S332 in Drosophila melanogaster[66]) are centromeric proteins which are essential to maintain cohesin bound to centromeres until anaphase. The human homolog, hsSgo1, associates with centromeres during prophase and disappears when anaphase starts.[67] When Shugoshin levels are reduced by RNAi in HeLa cells, cohesin cannot remain on the centromeres during mitosis, and consequently sister chromatids separate synchronically before anaphase initiates, which triggers a long mitotic arrest.
On the other hand, Dasso and collaborators have found that proteins involved in the Ran cycle can be detected on kinetochores during mitosis: RanGAP1 (a GTPase activating protein which stimulates the conversion of Ran-GTP in Ran-GDP) and the Ran binding protein called RanBP2/Nup358.[68] During interphase, these proteins are located at the nuclear pores and participate in the nucleo-cytoplasmic transport. Kinetochore localization of these proteins seem to be functionally significant, because some treatments that increase the levels of Ran-GTP inhibit kinetochore release of Bub1, Bub3, Mad2 and CENP-E.[69]
Orc2 (a protein that belongs to the origin recognition complex -ORC- implicated in DNA replication initiation during S phase) is also localized at kinetochores during mitosis in human cells;[70] in agreement with this localization, some studies indicate that Orc2 in yeast is implicated in sister chromatids cohesion, and when it is eliminated from the cell, spindle checkpoint activation ensues.[71] Some other ORC components (such orc5 in S. pombe) have been also found to participate in cohesion.[72] However, ORC proteins seem to participate in a molecular pathway which is additive to cohesin pathway, and it is mostly unknown.
Force generation to propel chromosome movement
Most chromosome movements in relation to spindle poles are associated to lengthening and shortening of kMTs. One of the most interesting features of kinetochores is their capacity to modify the state of their associated kMTs (around 20) from a depolymerization state at their (+) end to polymerization state. This allows the kinetochores from cells at prometaphase to show "directional instability",[73] changing between persistent phases of movement towards the pole (poleward) or inversed (anti-poleward), which are coupled with alternating states of kMTs depolymerization and polymerization, respectively. This kinetochore bi-stability seem to be part of a mechanism to align the chromosomes at the equator of the spindle without losing the mechanic connection between kinetochores and spindle poles. It is thought that kinetochore bi-stability is based upon the dynamic instability of the kMTs (+) end, and it is partially controlled by the tension present at the kinetochore. In mammalian cultured cells, a low tension at kinetochores promotes change towards kMTs depolymerization, and high tension promotes change towards kMTs polymerization.[74][75]
Kinetochore proteins and proteins binding to MTs (+) end (collectively called +TIPs) regulate kinetochore movement through the kMTs (+) end dynamics regulation.[76] However, the kinetochore-microtubule interface is highly dynamic, and some of these proteins seem to be bona fide components of both structures. Two groups of proteins seem to be particularly important: kinesins which work like depolymerases, such as KinI kinesins; and proteins bound to MT (+) ends, +TIPs, promoting polymerization, perhaps antagonizing the depolymerases effect.[77]
- KinI kinesins are named "I" because they present an internal motor domain, which uses ATP to promote depolymerization of tubulin polymer, the microtubule. In vertebrates, the most important KinI kinesin controlling the dynamics of the (+) end assembly is MCAK.[78] However, it seems that there are other kinesins implicated.
- There are two groups of +TIPs with kinetochore functions.
- The first one includes the protein adenomatous polyposis coli (APC) and the associated protein EB1, which need MTs to localize on the kinetochores. Both proteins are required for correct chromosome segregation.[79] EB1 binds only to MTs in polymerizing state, suggesting that it promotes kMTs stabilization during this phase.
- The second group of +TIPs includes proteins which can localize on kinetochores even in absence of MTs. In this group there are two proteins that have been widely studied: CLIP-170 and their associated proteins CLASPs (CLIP-associated proteins). CLIP-170 role at kinetochores is unknown, but the expression of a dominant negative mutant produces a prometaphase delay,[80] suggesting that it has an active role in chromosome alignment. CLASPs proteins are required for chromosome alignment and maintenance of a bipolar spindle in Drosophila, humans and yeast.[81][82]
References
- ↑ Brooker, Robert J. (2016). Concepts of Genetics. New York: McGraw Hill Education.
- ↑ Albertson, D.G.; Thomson, J.N. (1993), "Segregation of holocentric chromosomes at meiosis in the nematode, Caenorhabditis elegans", Chromosome Research, 1 (1): 15–26, doi:10.1007/BF00710603, PMID 8143084
- ↑ Peter De Wulf, William C. Earnshaw, The Kinetochore: From Molecular Discoveries to Cancer Therapy
- 1 2 Maiato, H.; Deluca, J.; Salmon, E.D.; Earnshaw, W.C. (2004), "The dynamic kinetochore-microtubule interface", Journal of Cell Science, 117 (22): 5461–5477, doi:10.1242/jcs.01536, PMID 15509863
- ↑ Mitchison, T.; Kirschner, M. (1984), "Dynamic instability of microtubule growth" (PDF), Nature, 312 (5991): 237–242, doi:10.1038/312237a0, PMID 6504138
- 1 2 Brinkley, B.R.; Stubblefield, E. (1966), "The fine structure of the kinetochore of a mammalian cell in vitro", Chromosoma, 19 (1): 28–43, doi:10.1007/BF00332792, PMID 5912064
- ↑ Jokelainen, P.T. (1967), "The ultrastructure and spatial organization of the metaphase kinetochore in mitotic rat cells", J Ultrastruct Res, 19 (1): 19–44, doi:10.1016/S0022-5320(67)80058-3, PMID 5339062
- ↑ Rieder, C.L. (1982), "The formation, structure, and composition of the mammalian kinetochore and kinetochore fiber", Int Rev Cytol, International Review of Cytology, 79: 1–58, doi:10.1016/S0074-7696(08)61672-1, ISBN 978-0-12-364479-4, PMID 6185450
- ↑ McEwen, B.F.; Hsieh, C.E.; Mattheyses, A.L.; Rieder, C.L. (1998), "A new look at kinetochore structure in vertebrate somatic cells using high-pressure freezing and freeze substitution", Chromosoma, 107 (6): 366–375, doi:10.1007/s004120050320, PMC 2905855
 , PMID 9914368
, PMID 9914368 - ↑ Brenner, S.; Pepper, D.; Berns, M.W.; Tan, E.; Brinkley, B.R. (1981), "Kinetochore structure, duplication, and distribution in mammalian cells: analysis by human autoantibodies from scleroderma patients", The Journal of Cell Biology, 91 (1): 95–102, doi:10.1083/jcb.91.1.95, PMC 2111947
 , PMID 7298727
, PMID 7298727 - ↑ Pluta, A.F.; MacKay, A.M.; Ainsztein, A.M.; Goldberg, I.G.; Earnshaw, W.C. (1995), "The Centromere: Hub of Chromosomal Activities", Science, 270 (5242): 1591–4, doi:10.1126/science.270.5242.1591, PMID 7502067
- ↑ Palmer, D.K.; O'Day, K.; Trong, H.L.; Charbonneau, H.; Margolis, R.L. (1991), "Purification of the centromere-specific protein CENP-A and demonstration that it is a distinctive histone", Proceedings of the National Academy of Sciences, 88 (9): 3734–3738, doi:10.1073/pnas.88.9.3734, PMC 51527
 , PMID 2023923
, PMID 2023923 - ↑ Howman, E.V.; Fowler, K.J.; Newson, A.J.; Redward, S.; MacDonald, A.C.; Kalitsis, P.; Choo, K.H.A. (2000), "Early disruption of centromeric chromatin organization in centromere protein A (Cenpa) null mice", Proceedings of the National Academy of Sciences, 97 (3): 1148–1153, doi:10.1073/pnas.97.3.1148
- ↑ Oegema, K.; Desai, A.; Rybina, S.; Kirkham, M.; Hyman, A.A. (2001), "Functional Analysis of Kinetochore Assembly in Caenorhabditis elegans", The Journal of Cell Biology, 153 (6): 1209–1226, doi:10.1083/jcb.153.6.1209, PMC 2192036
 , PMID 11402065
, PMID 11402065 - ↑ Van Hooser, A.A.; Ouspenski, I.I.; Gregson, H.C.; Starr, D.A.; Yen, T.J.; Goldberg, M.L.; Yokomori, K.; Earnshaw, W.C.; Sullivan, K.F. (2001), "Specification of kinetochore-forming chromatin by the histone H3 variant CENP-A", Journal of Cell Science, 114 (19): 3529–3542, PMID 11682612
- ↑ Fukagawa, T.; Mikami, Y.; Nishihashi, A.; Regnier, V.; Haraguchi, T.; Hiraoka, Y.; Sugata, N.; Todokoro, K.; Brown, W. (2001), "CENP-H, a constitutive centromere component, is required for centromere targeting of CENP-C in vertebrate cells", The EMBO Journal, 20 (16): 4603–4617, doi:10.1093/emboj/20.16.4603, PMC 125570
 , PMID 11500386
, PMID 11500386 - ↑ Goshima, G.; Kiyomitsu, T.; Yoda, K.; Yanagida, M. (2003), "Human centromere chromatin protein hMis12, essential for equal segregation, is independent of CENP-A loading pathway", The Journal of Cell Biology, 160 (1): 25–39, doi:10.1083/jcb.200210005, PMC 2172742
 , PMID 12515822
, PMID 12515822 - 1 2 3 4 Wigge, Philip A.; Kilmartin, John V. (2001), "The Ndc80p Complex from Saccharomyces cerevisiae Contains Conserved Centromere Components and Has a Function in Chromosome Segregation", The Journal of Cell Biology, 152 (2): 349–360, doi:10.1083/jcb.152.2.349, PMC 2199619
 , PMID 11266451
, PMID 11266451 - 1 2 3 4 Deluca, J.G.; Moree, B.; Hickey, J.M.; Kilmartin, J.V.; Salmon, E.D. (2002), "hNuf2 inhibition blocks stable kinetochore–microtubule attachment and induces mitotic cell death in HeLa cells", The Journal of Cell Biology, 159 (4): 549–555, doi:10.1083/jcb.200208159, PMC 2173110
 , PMID 12438418
, PMID 12438418 - 1 2 Cheeseman, I.M.; Niessen, S.; Anderson, S.; Hyndman, F.; Yates, J.R.; Oegema, K.; Desai, A. (2004), "A conserved protein network controls assembly of the outer kinetochore and its ability to sustain tension", Genes & Development, 18 (18): 2255–2268, doi:10.1101/gad.1234104, PMC 517519
 , PMID 15371340
, PMID 15371340 - ↑ Rattner, J.B.; Rao, A.; Fritzler, M.J.; Valencia, D.W.; Yen, T.J. (1993), "CENP-F is a. Ca 400 kDa kinetochore protein that exhibits a cell-cycle dependent localization", Cell Motil Cytoskeleton, 26 (3): 214–26, doi:10.1002/cm.970260305, PMID 7904902
- ↑ Liao, H.; Winkfein, RJ; Mack, G; Rattner, JB; Yen, TJ (1995), "CENP-F is a protein of the nuclear matrix that assembles onto kinetochores at late G2 and is rapidly degraded after mitosis", The Journal of Cell Biology, 130 (3): 507–518, doi:10.1083/jcb.130.3.507, PMC 2120529
 , PMID 7542657
, PMID 7542657 - 1 2 3 4 5 Hoffman, DB; Hoffman, D.B.; Pearson, C.G.; Yen, T.J.; Howell, B.J.; Salmon, E.D. (2001), "Microtubule-dependent changes in assembly of microtubule motor proteins and mitotic spindle checkpoint proteins at PtK1 kinetochores", Molecular Biology of the Cell, 12 (7): 1995–2009, doi:10.1091/mbc.12.7.1995, PMC 55648
 , PMID 11451998
, PMID 11451998 - 1 2 King, S.M. (2000), "The dynein microtubule motor", BBA Molecular Cell Research, 1496 (1): 60–75, doi:10.1016/S0167-4889(00)00009-4, PMID 10722877
- 1 2 Howell, B.J.; Moree, B.; Farrar, E.M.; Stewart, S.; Fang, G.; Salmon, E.D. (2004), "Spindle Checkpoint Protein Dynamics at Kinetochores in Living Cells", Current Biology, 14 (11): 953–964, doi:10.1016/j.cub.2004.05.053, PMID 15182668
- 1 2 3 Shah, J.V.; Botvinick, E.; Bonday, Z.; Furnari, F.; Berns, M.; Cleveland, D.W. (2004), "Dynamics of Centromere and Kinetochore Proteins Implications for Checkpoint Signaling and Silencing", Current Biology, 14 (11): 942–952, doi:10.1016/j.cub.2004.05.046, PMID 15182667
- ↑ Tirnauer, Jennifer S.; Canman, Julie C.; Salmon, E.D.; Mitchison, Timothy J. (2002), "EB1 Targets to Kinetochores with Attached, Polymerizing Microtubules", Molecular Biology of the Cell, 13 (12): 4308–4316, doi:10.1091/mbc.E02-04-0236, PMC 138635
 , PMID 12475954
, PMID 12475954 - ↑ Kaplan, K.B.; Burds, A.A.; Swedlow, J.R.; Bekir, S.S.; Sorger, P.K.; Näthke, I.S. (2001), "A role for the Adenomatous Polyposis Coli protein in chromosome segregation", Nature Cell Biology, 3 (4): 429–432, doi:10.1038/35070123, PMID 11283619
- ↑ Joseph, J.; Liu, S.T.; Jablonski, S.A.; Yen, T.J.; Dasso, M. (2004), "The RanGAP1-RanBP2 Complex is Essential for Microtubule-Kinetochore Interactions in Vivo", Current Biology, 14 (7): 611–617, doi:10.1016/j.cub.2004.03.031, PMID 15062103
- ↑ Salina, Davide; Enarson, Paul; Rattner, J.B.; Burke, Brian (2003), "Nup358 integrates nuclear envelope breakdown with kinetochore assembly", The Journal of Cell Biology, 162 (6): 991–1002, doi:10.1083/jcb.200304080, PMC 2172838
 , PMID 12963708
, PMID 12963708 - ↑ Ohta S, Bukowski-Wills JC, Sanchez-Pulido L, Alves Fde L, Wood L, Chen ZA, Platani M, Fischer L, Hudson DF, Ponting CP, Fukagawa T, Earnshaw WC, Rappsilber J (September 2010), "The Protein Composition of Mitotic Chromosomes Determined Using Multiclassifier Combinatorial Proteomics", Cell, 142 (5): 810–21, doi:10.1016/j.cell.2010.07.047, PMC 2982257
 , PMID 20813266
, PMID 20813266 - ↑ Tipton AR, Wang K, Oladimeji P, Sufi S, Gu Z, Liu ST (2012), "Identification of novel mitosis regulators through data mining with human centromere/kinetochore proteins as group queries", BMC Cell Biol, 13: 15, doi:10.1186/1471-2121-13-15, PMID 22712476
- ↑ McEwen, B.F.; Heagle, A.B.; Cassels, G.O.; Buttle, K.F.; Rieder, C.L. (1997), "Kinetochore Fiber Maturation in PtK1 Cells and Its Implications for the Mechanisms of Chromosome Congression and Anaphase Onset", The Journal of Cell Biology, 137 (7): 1567–1580, doi:10.1083/jcb.137.7.1567, PMC 2137823
 , PMID 9199171
, PMID 9199171 - 1 2 Nicklas, R.B.; Kubai, D.F. (1985), "Microtubules, chromosome movement, and reorientation after chromosomes are detached from the spindle by micromanipulation", Chromosoma, 92 (4): 313–324, doi:10.1007/BF00329815, PMID 4042772
- ↑ Mayor, T.; Meraldi, P.; Stierhof, Y.D.; Nigg, E.A.; Fry, A.M. (1999), "Protein kinases in control of the centrosome cycle", FEBS Letters, 452 (1–2): 92–95, doi:10.1016/S0014-5793(99)00534-7, PMID 10376685
- 1 2 Kirschner, M.; Mitchison, T. (1986), "Beyond self-assembly: from microtubules to morphogenesis", Cell, 45 (3): 329–342, doi:10.1016/0092-8674(86)90318-1, PMID 3516413
- ↑ Holy, T. E.; Leibler, S. (1994), "Dynamic instability of microtubules as an efficient way to search in space", Proceedings of the National Academy of Sciences of the United States of America, 91 (12): 5682–5685, doi:10.1073/pnas.91.12.5682, PMC 44060
 , PMID 8202548
, PMID 8202548 - ↑ Hayden, J.H.; Bowser, SS; Rieder, CL (1990), "Kinetochores capture astral microtubules during chromosome attachment to the mitotic spindle: direct visualization in live newt lung cells", The Journal of Cell Biology, 111 (3): 1039–1045, doi:10.1083/jcb.111.3.1039, PMC 2116290
 , PMID 2391359
, PMID 2391359 - ↑ Nicklas, R.B. (1997), "How Cells Get the Right Chromosomes", Science, 275 (5300): 632–7, doi:10.1126/science.275.5300.632, PMID 9005842
- ↑ Loncarek, J.; Kisurina-evgenieva, O.; Vinogradova, T.; Hergert, P.; La Terra, S.; Kapoor, T.M.; Khodjakov, A. (2007), "The centromere geometry essential for error-free mitosis is controlled by spindle forces", Nature, 450 (7170): 745–9, doi:10.1038/nature06344, PMC 2586812
 , PMID 18046416
, PMID 18046416 - ↑ Dewar, H.; Tanaka, K.; Nasmyth, K.; Tanaka, T.U. (2004), "Tension between two kinetochores suffices for their bi-orientation on the mitotic spindle", Nature, 428 (6978): 93–7, doi:10.1038/nature02328, PMID 14961024
- ↑ Echeverri, C.J.; Paschal, B.M.; Vaughan, K.T.; Vallee, R.B. (1996), "Molecular characterization of the 50-kD subunit of dynactin reveals function for the complex in chromosome alignment and spindle organization during mitosis", The Journal of Cell Biology, 132 (4): 617–633, doi:10.1083/jcb.132.4.617, PMC 2199864
 , PMID 8647893
, PMID 8647893 - ↑ Sharp, D.J.; Rogers, G.C.; Scholey, J.M. (2000), "Cytoplasmic dynein is required for poleward chromosome movement during mitosis in Drosophila embryos", Nature Cell Biology, 2 (12): 922–930, doi:10.1038/35046574, PMID 11146657
- ↑ Banks, J.D.; Heald, R. (2001), "Chromosome movement: Dynein-out at the kinetochore", Current Biology, 11 (4): 128–131, doi:10.1016/S0960-9822(01)00059-8
- ↑ Howell, B.J.; McEwen, B.F.; Canman, J.C.; Hoffman, D.B.; Farrar, E.M.; Rieder, C.L.; Salmon, E.D. (2001), "Cytoplasmic dynein/dynactin drives kinetochore protein transport to the spindle poles and has a role in mitotic spindle checkpoint inactivation", The Journal of Cell Biology, 155 (7): 1159–1172, doi:10.1083/jcb.200105093, PMC 2199338
 , PMID 11756470
, PMID 11756470 - ↑ Cooke, C.A.; Schaar, B.; Yen, T.J.; Earnshaw, W.C. (1997), "LLocalization of CENP-E in the fibrous corona and outer plate of mammalian kinetochores from prometaphase through anaphase", Chromosoma, 106 (7): 446–455, doi:10.1007/s004120050266, PMID 9391217
- ↑ Weaver, Beth A.A.; Bonday, Zahid Q.; Putkey, Frances R.; Kops, Geert J.P.L.; Silk, Alain D.; Cleveland, Don W. (2003), "Centromere-associated protein-E is essential for the mammalian mitotic checkpoint to prevent aneuploidy due to single chromosome loss", The Journal of Cell Biology, 162 (4): 551–563, doi:10.1083/jcb.200303167, PMC 2173788
 , PMID 12925705
, PMID 12925705 - 1 2 Maiato, H.; Rieder, C.L.; Khodjakov, A. (2004), "Kinetochore-driven formation of kinetochore fibers contributes to spindle assembly during animal mitosis", The Journal of Cell Biology, 167 (5): 831–840, doi:10.1083/jcb.200407090, PMC 2172442
 , PMID 15569709
, PMID 15569709 - ↑ Mitchison, T.J. (1988), "Microtubule Dynamics and Kinetochore Function in Mitosis", Annual Review of Cell Biology, 4 (1): 527–545, doi:10.1146/annurev.cb.04.110188.002523
- 1 2 3 He, X.; Rines, D.R.; Espelin, C.W.; Sorger, P.K. (2001), "Molecular Analysis of Kinetochore-Microtubule Attachment in Budding Yeast", Cell, 106 (2): 195–206, doi:10.1016/S0092-8674(01)00438-X, PMID 11511347
- 1 2 Westermann, Stefan; Cheeseman, Iain M.; Anderson, Scott; Yates, John R.; I. I. I., DG; Drubin, David G.; Barnes, Georjana (2003), "Architecture of the budding yeast kinetochore reveals a conserved molecular core", The Journal of Cell Biology, 163 (2): 215–22, doi:10.1083/jcb.200305100, PMC 2173538
 , PMID 14581449
, PMID 14581449 - 1 2 De Wulf, P.; McAinsh, A.D.; Sorger, P.K. (2003), "Hierarchical assembly of the budding yeast kinetochore from multiple subcomplexes", Genes & Development, 17 (23): 2902–2921, doi:10.1101/gad.1144403
- ↑ Goh, P.Y.; Kilmartin, J.V. (1993), "NDC10: a gene involved in chromosome segregation in Saccharomyces cerevisiae", The Journal of Cell Biology, 121 (3): 503–12, doi:10.1083/jcb.121.3.503, PMC 2119568
 , PMID 8486732
, PMID 8486732 - ↑ Nabetani, A.; Koujin, T.; Tsutsumi, C.; Haraguchi, T.; Hiraoka, Y. (2001), "A conserved protein, Nuf2, is implicated in connecting the centromere to the spindle during chromosome segregation: a link between the kinetochore function and the spindle checkpoint", Chromosoma, 110 (5): 322–334, doi:10.1007/s004120100153, PMID 11685532
- 1 2 Howe, Mary; McDonald, Kent L.; Albertson, Donna G.; Meyer, Barbara J. (2001), "Him-10 Is Required for Kinetochore Structure and Function on Caenorhabditis elegans Holocentric Chromosomes", The Journal of Cell Biology, 153 (6): 1227–1238, doi:10.1083/jcb.153.6.1227, PMC 2192032
 , PMID 11402066
, PMID 11402066 - 1 2 3 Martin-lluesma, Silvia; Stucke, Volker M.; Nigg, Erich A. (2002), "Role of Hec1 in Spindle Checkpoint Signaling and Kinetochore Recruitment of Mad1/Mad2", Science, 297 (5590): 2267–2270, doi:10.1126/science.1075596, PMID 12351790
- 1 2 3 McCleland, M.L.; Gardner, R.D.; Kallio, M.J.; Daum, J.R.; Gorbsky, G.J.; Burke, D.J.; Stukenberg, P.T. (2003), "The highly conserved Ndc80 complex is required for kinetochore assembly, chromosome congression, and spindle checkpoint activity", Genes & Development, 17 (1): 101–114, doi:10.1101/gad.1040903
- ↑ Zheng, L.; Chen, Y.; Lee, W.H. (1999), "Hec1p, an Evolutionarily Conserved Coiled-Coil Protein, Modulates Chromosome Segregation through Interaction with SMC Proteins", Molecular and Cellular Biology, 19 (8): 5417–5428, doi:10.1128/mcb.19.8.5417, PMC 84384
 , PMID 10409732
, PMID 10409732 - ↑ Wei, Ronnie R.; Al-bassam, Jawdat; Harrison, Stephen C. (2007), "The Ndc80/HEC1 complex is a contact point for kinetochore-microtubule attachment", Nature Structural & Molecular Biology, 14 (1): 54–59, doi:10.1038/nsmb1186
- ↑ Courtwright, A.M.; He, X. (2002), "Dam1 is the Right One Phosphoregulation of Kinetochore Biorientation", Developmental Cell, 3 (5): 610–611, doi:10.1016/S1534-5807(02)00332-5, PMID 12431367
- 1 2 Cimini, D.; Moree, B.; Canman, J.C.; Salmon, E.D. (2003), "Merotelic kinetochore orientation occurs frequently during early mitosis in mammalian tissue cells and error correction is achieved by two different mechanisms", Journal of Cell Science, 116 (20): 4213–4225, doi:10.1242/jcs.00716
- ↑ Adams, R.R.; Carmena, M.; Earnshaw, W.C. (2001), "Chromosomal passengers and the (aurora) ABCs of mitosis", Trends in Cell Biology, 11 (2): 49–54, doi:10.1016/S0962-8924(00)01880-8, PMID 11166196
- ↑ Cheeseman, I.M.; Anderson, S.; Jwa, M.; Green, E.M.; Kang, J.; Yates, J.R.; Chan, C.S.M.; Drubin, D.G.; Barnes, G. (2002), "Phospho-Regulation of Kinetochore-Microtubule Attachments by the Aurora Kinase Ipl1p", Cell, 111 (2): 163–172, doi:10.1016/S0092-8674(02)00973-X, PMID 12408861
- ↑ Gautschi, Oliver; Heighway, Jim; Mack, Philip C.; Purnell, Phillip R.; Lara, Primo N.; Jr, .; Gandara, David R. (2008), "Aurora Kinases as Anticancer Drug Targets", Clinical Cancer Research, 14 (6): 1639–48, doi:10.1158/1078-0432.CCR-07-2179, PMID 18347165
- ↑ Meraldi, P.; Draviam, V.M.; Sorger, P.K. (2004), "Timing and Checkpoints in the Regulation of Mitotic Progression", Developmental Cell, 7 (1): 45–60, doi:10.1016/j.devcel.2004.06.006, PMID 15239953
- ↑ Tang, T.T.L.; Bickel, S.E.; Young, L.M.; Orr-weaver, T.L. (1998), "Maintenance of sister-chromatid cohesion at the centromere by the Drosophila MEI-S332 protein", Genes & Development, 12 (24): 3843–3856, doi:10.1101/gad.12.24.3843
- ↑ McGuinness, B.E.; Hirota, T.; Kudo, N.R.; Peters, J.M.; Nasmyth, K. (2005), "Shugoshin Prevents Dissociation of Cohesin from Centromeres During Mitosis in Vertebrate Cells", PLoS Biol, 3 (3): e86, doi:10.1371/journal.pbio.0030086, PMC 1054882
 , PMID 15737064
, PMID 15737064 - ↑ Joseph, Jomon; Tan, Shyh-Han; Karpova, Tatiana S.; McNally, James G.; Dasso, Mary (2002), "SUMO-1 targets RanGAP1 to kinetochores and mitotic spindles", The Journal of Cell Biology, 156 (4): 595–602, doi:10.1083/jcb.200110109, PMC 2174074
 , PMID 11854305
, PMID 11854305 - ↑ Arnaoutov, A.; Dasso, M. (2003), "The Ran GTPase Regulates Kinetochore Function", Developmental Cell, 5 (1): 99–111, doi:10.1016/S1534-5807(03)00194-1, PMID 12852855
- ↑ Prasanth, S.G.; Prasanth, K.V.; Siddiqui, K.; Spector, D.L.; Stillman, B. (2004), "Human Orc2 localizes to centrosomes, centromeres and heterochromatin during chromosome inheritance", The EMBO Journal, 23 (13): 2651–2663, doi:10.1038/sj.emboj.7600255, PMC 449767
 , PMID 15215892
, PMID 15215892 - ↑ Shimada, K.; Gasser, S.M. (2007), "The Origin Recognition Complex Functions in Sister-Chromatid Cohesion in Saccharomyces cerevisiae", Cell, 128 (1): 85–99, doi:10.1016/j.cell.2006.11.045, PMID 17218257
- ↑ Kato, H; Matsunaga, F; Miyazaki, S; Yin, L; D'urso, G; Tanaka, K; Murakami, Y (2008), "Schizosaccharomyces pombe Orc5 plays multiple roles in the maintenance of genome stability throughout the cell cycle", Cell cycle, 7 (8): 1085–96, doi:10.4161/cc.7.8.5710, PMID 18414064
- ↑ Skibbens, R.V.; Skeen, V.P.; Salmon, E.D. (1993), "Directional instability of kinetochore motility during chromosome congression and segregation in mitotic newt lung cells: a push-pull mechanism", The Journal of Cell Biology, 122 (4): 859–875, doi:10.1083/jcb.122.4.859, PMC 2119582
 , PMID 8349735
, PMID 8349735 - ↑ Rieder, C.L.; Salmon, E.D. (1994), "Motile kinetochores and polar ejection forces dictate chromosome position on the vertebrate mitotic spindle", The Journal of Cell Biology, 124 (3): 223–33, doi:10.1083/jcb.124.3.223, PMC 2119939
 , PMID 8294508
, PMID 8294508 - ↑ Skibbens, RV; Rieder, CL; Salmon, ED (1995), "Kinetochore motility after severing between sister centromeres using laser microsurgery: evidence that kinetochore directional instability and position is regulated by tension", Journal of Cell Science, 108 (7): 2537–48, PMID 7593295
- ↑ Askham, J. M.; Vaughan, K. T.; Goodson, H. V.; Morrison, E. E. (2002), "Evidence That an Interaction between EB1 and p150Glued Is Required for the Formation and Maintenance of a Radial Microtubule Array Anchored at the Centrosome", Molecular Biology of the Cell, 13 (10): 3627–3645, doi:10.1091/mbc.E02-01-0061, PMC 129971
 , PMID 12388762
, PMID 12388762 - ↑ Schuyler, S.C.; Pellman, D. (2001), "Microtubule "Plus-End-Tracking Proteins" the End is Just the Beginning", Cell, 105 (4): 421–424, doi:10.1016/S0092-8674(01)00364-6, PMID 11371339
- ↑ Howard, J.; Hyman, A.A. (2003), "Dynamics and mechanics of the microtubule plus end: cytoskeleton", Nature, 422 (6933): 753–758, doi:10.1038/nature01600, PMID 12700769
- ↑ Green, R.A.; Wollman, R.; Kaplan, K.B. (2005), "APC and EB1 Function Together in Mitosis to Regulate Spindle Dynamics and Chromosome Alignment", Molecular Biology of the Cell, 16 (10): 4609–4622, doi:10.1091/mbc.E05-03-0259, PMC 1237068
 , PMID 16030254
, PMID 16030254 - ↑ Dujardin, D.; Wacker, U.I.; Moreau, A.; Schroer, T.A.; Rickard, J.E.; De Mey, J.R. (1998), "Evidence for a Role of CLIP-170 in the Establishment of Metaphase Chromosome Alignment", The Journal of Cell Biology, 141 (4): 849–862, doi:10.1083/jcb.141.4.849, PMC 2132766
 , PMID 9585405
, PMID 9585405 - ↑ Maiato, H.; Khodjakov, A.; Rieder, C.L. (2004), "Drosophila CLASP is required for the incorporation of microtubule subunits into fluxing kinetochore fibres", Nature Cell Biology, 7 (1): 42–47, doi:10.1038/ncb1207, PMC 2596653
 , PMID 15592460
, PMID 15592460 - ↑ Maiato, H.; Fairley, E.A.L.; Rieder, C.L.; Swedlow, J.R.; Sunkel, C.E.; Earnshaw, W.C. (2003), "Human CLASP1 is an Outer Kinetochore Component that Regulates Spindle Microtubule Dynamics", Cell, 113 (7): 891–904, doi:10.1016/S0092-8674(03)00465-3, PMID 12837247