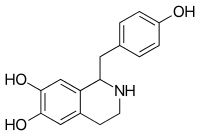
Higenamine
 | |
| Names | |
|---|---|
| IUPAC name
1-[(4-Hydroxyphenyl)methyl]-1,2,3,4-tetrahydroisoquinoline-6,7-diol | |
| Other names
norcoclaurine, demethylcoclaurine | |
| Identifiers | |
| 5843-65-2 106032-53-5 (R) 22672-77-1 (S) | |
| 3D model (Jmol) | Interactive image |
| ChEBI | CHEBI:18418 |
| ChEMBL | ChEMBL19344 |
| ChemSpider | 102800 |
| KEGG | C06346 |
| MeSH | higenamine |
| PubChem | 114840 |
| |
| |
| Properties | |
| C16H17NO3 | |
| Molar mass | 271.32 g·mol−1 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Higenamine (norcoclaurine) is a chemical compound found in a variety of plants including Nandina domestica (fruit), Aconitum carmichaelii (root), Asarum heterotropioides, Galium divaricatum (stem and vine), Annona squamosa, and Nelumbo nucifera (lotus seeds).
Legality
Higenamine, also known as norcoclaurine HCl, is legal to use within food supplements in the UK, EU, the USA and Canada. Its main use is within food supplements developed for weight management, also known as 'fat burners'. Traditional formulations with higenamine have been used for thousands of years within Chinese medicine and come from a variety of sources including fruit and orchids. There are no studies comparing the safety of modern formulations (based on synthetic higenamine) with traditional formulations. Nevertheless, it will not be added to the EU 'novel foods' catalogue, which details all food supplements that require a safety assessment certificate before use.[1]
Along with many other β2 agonists, higenamine is prohibited by World Anti-Doping Agency for use in sports.[2] In 2016, French footballer Mamadou Sakho was temporarily banned by UEFA after testing positive for Higenamine causing the player to miss the 2016 Europa League final. The ban was lifted after the player successfully made the mitigating defence that there was an absence of significant negligence as the substance was not on the list of banned substances despite drugs of the same category – β2 agonists – being banned.[3][4][5][6]
Pharmacology
Since higenamine is present in plants which have a history of use in traditional medicine, the pharmacology of this compound has attracted scientific interest. A variety of effects have been observed in in vitro studies and in animal models, but its effects in humans are unknown.
In animal models, higenamine has been demonstrated to be a β2 adrenoreceptor agonist.[7][8][9][10][11] Adrenergic receptors, or adrenoceptors, belong to the class of G protein–coupled receptors, and are the most prominent receptors in the adipose membrane, besides also being expressed in skeletal muscle tissue. These adipose membrane receptors are classified as either α or β adrenoceptors. Although these adrenoceptors share the same messenger, cyclic adenosine monophosphate (cAMP), the specific transduction pathway depends on the receptor type (α or β). Higenamine partly exerts its actions by the activation of an enzyme, adenylate cyclase, responsible for boosting the cellular concentrations of the adrenergic second messenger, cAMP.[12]
In a rodent model, it was found that higenamine produced cardiotonic, vascular relaxation, and bronchodilator effects.[13][14] In particular, higenamine, via a beta-adrenoceptor mechanism, induced relaxation in rat corpus cavernosum, leading to improved vasodilation and erectile function.
Related to improved vasodilatory signals, higenamine has been shown in animal models to possess antiplatelet and antithrombotic activity via a cAMP-dependent pathway, suggesting higenamine may contribute to enhanced vasodilation and arterial integrity.[7][12][14][15]
Toxicity
Regarding toxicity, researchers have suggested that the levels of higenamine reported in food consumption (estimated 47.5 mg in a 9-ounce serving of Lotus) would be comparable to the amount used in food supplements.[16] Higenamine is a beta-adrenergic agonist which has effects on the function of trachea and heart muscles.[17][18] During a study of acute toxicity, mice were orally administered the compound at a dose of 2 g per kg of bodyweight. No mice died during the study.[19]
See also
References
- ↑ http://ec.europa.eu/food/food/biotechnology/novelfood/novel_food_catalogue_en.htm
- ↑ "Prohibited Substances at All Times". List of Prohibited Substances and Methods. World Anti-Doping Agency. 1 January 2016. Retrieved 21 August 2016.
- ↑ http://www.bbc.co.uk/sport/football/36120459
- ↑ http://www.bbc.co.uk/sport/football/36406071
- ↑ http://www.liverpoolecho.co.uk/sport/football/football-news/mamadou-sakho-uefa-decision-raises-11399116
- ↑ http://www.getfootballnewsfrance.com/2016/mamadou-sakho-still-set-to-miss-euro-2016-despite-being-cleared-of-doping/
- 1 2 Tsukiyama, M; Ueki, T; Yasuda, Y; Kikuchi, H; Akaishi, T; Okumura, H; Abe, K (2009). "Beta2-adrenoceptor-mediated tracheal relaxation induced by higenamine from Nandina domestica Thunberg". Planta Medica. 75 (13): 1393–9. doi:10.1055/s-0029-1185743. PMID 19468973.
- ↑ Kashiwada, Y; Aoshima, A; Ikeshiro, Y; Chen, YP; Furukawa, H; Itoigawa, M; Fujioka, T; Mihashi, K; et al. (2005). "Anti-HIV benzylisoquinoline alkaloids and flavonoids from the leaves of Nelumbo nucifera, and structure-activity correlations with related alkaloids". Bioorganic & Medicinal Chemistry. 13 (2): 443–8. doi:10.1016/j.bmc.2004.10.020. PMID 15598565.
- ↑ Kimura, I; Chui, LH; Fujitani, K; Kikuchi, T; Kimura, M (1989). "Inotropic effects of (+/-)-higenamine and its chemically related components, (+)-R-coclaurine and (+)-S-reticuline, contained in the traditional sino-Japanese medicines "bushi" and "shin-i" in isolated guinea pig papillary muscle". Japanese journal of pharmacology. 50 (1): 75–8. doi:10.1254/jjp.50.75. PMID 2724702.
- ↑ Kang, YJ; Lee, YS; Lee, GW; Lee, DH; Ryu, JC; Yun-Choi, HS; Chang, KC (1999). "Inhibition of activation of nuclear factor kappaB is responsible for inhibition of inducible nitric oxide synthase expression by higenamine, an active component of aconite root". The Journal of Pharmacology and Experimental Therapeutics. 291 (1): 314–20. PMID 10490919.
- ↑ Yun-Choi, HS; Pyo, MK; Park, KM; Chang, KC; Lee, DH (2001). "Anti-thrombotic effects of higenamine". Planta Medica. 67 (7): 619–22. doi:10.1055/s-2001-17361. PMID 11582538.
- 1 2 Kam, SC; Do, JM; Choi, JH; Jeon, BT; Roh, GS; Chang, KC; Hyun, JS (2012). "The relaxation effect and mechanism of action of higenamine in the rat corpus cavernosum". International Journal of Impotence Research. 24 (2): 77–83. doi:10.1038/ijir.2011.48. PMID 21956762.
- ↑ Bai, G; Yang, Y; Shi, Q; Liu, Z; Zhang, Q; Zhu, YY (2008). "Identification of higenamine in Radix Aconiti Lateralis Preparata as a beta2-adrenergic receptor agonist1". Acta pharmacologica Sinica. 29 (10): 1187–94. doi:10.1111/j.1745-7254.2008.00859.x. PMID 18817623.
- 1 2 Pyo, MK; Lee, DH; Kim, DH; Lee, JH; Moon, JC; Chang, KC; Yun-Choi, HS (2008). "Enantioselective synthesis of (R)-(+)- and (S)-(-)-higenamine and their analogues with effects on platelet aggregation and experimental animal model of disseminated intravascular coagulation". Bioorganic & Medicinal Chemistry Letters. 18 (14): 4110–4. doi:10.1016/j.bmcl.2008.05.094. PMID 18556200.
- ↑ Liu, W; Sato, Y; Hosoda, Y; Hirasawa, K; Hanai, H (2000). "Effects of higenamine on regulation of ion transport in guinea pig distal colon". Japanese journal of pharmacology. 84 (3): 244–51. doi:10.1254/jjp.84.244. PMID 11138724.
- ↑ https://www.bulkhealthcapsules.com/products/phentramine-t9-rx-50mg?variant=709435407
- ↑ Wong, KK; Lo, CF; Chen, CM (1997). "Endothelium-dependent higenamine-induced aortic relaxation in isolated rat aorta". Planta Medica. 63 (2): 130–2. doi:10.1055/s-2006-957628. PMID 9140225.
- ↑ Ueki, T; Akaishi, T; Okumura, H; Morioka, T; Abe, K (2011). "Biphasic tracheal relaxation induced by higenamine and nantenine from Nandina domestica Thunberg". Journal of pharmacological sciences. 115 (2): 254–7. doi:10.1254/jphs.10251sc. PMID 21282929.
- ↑ Lo, CF; Chen, CM (1997). "Acute toxicity of higenamine in mice". Planta Medica. 63 (1): 95–6. doi:10.1055/s-2006-957619. PMID 9063102.