Hexoprenaline
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | Oral (tablets), IV |
| ATC code | R03AC06 (WHO) R03CC05 (WHO) |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 5–11% (Tmax = 2 hours) |
| Metabolism | COMT (slow O-methylation) |
| Biological half-life | ~50 minutes (if taken orally) |
| Excretion | Feces (~90%)[1] |
| Identifiers | |
| |
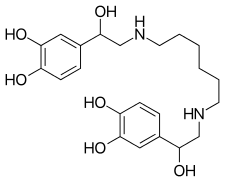
| Synonyms | 4-[2-[6-[[2-(3,4-dihydroxyphenyl)-2-hydroxyethyl]amino]hexylamino]-1-hydroxyethyl]benzene-1,2-diol |
| CAS Number |
3215-70-1 |
| PubChem (CID) | 3609 |
| DrugBank |
DB08957 |
| ChemSpider |
3483 |
| UNII |
G9L6B3W684 |
| KEGG |
D08039 |
| Chemical and physical data | |
| Formula | C22H32N2O6 |
| Molar mass | 420.499 g/mol |
| 3D model (Jmol) | Interactive image |
| Chirality | Racemic mixture |
| |
| |
| | |
Hexoprenaline (HEX-oh-PREN-a-leen, INN) is a selective β2 adrenergic receptor agonist used in the treatment of asthma.[2] Hexoprenaline is also used in some countries (such as Russia) as a tocolytic agent (i.e., labor suppressant), with the most common trade name being Gynipral.[3] It is not approved by U.S. FDA.
Contraindications
When used as a tocolytic, hexoprenaline is contraindicated in:
- Hyperthyroidism
- Cardiovascular diseases, e.g. cardiac arrhythmias, tachycardia, myocarditis, mitral valve disease and aortic stenosis
- Ischemic heart disease
- Hypertension
- Angle-closure glaucoma
- Placental abruption, vaginal bleeding and inflammatory diseases of internal genitalia (such as endometritis)
- Shock
- First trimester of pregnancy
- Breastfeeding[1][3]
It should be used with caution in people with gestational diabetes.
Drug-drug interactions
When concomitantly administered:
- Beta blockers reduce or neutralize therapeutic effects of hexoprenaline
- Methylxanthines (caffeine, theobromine, theophylline) increase its action
- General anaesthetics (e.g., halothane) and adrenergic receptor agonists may increase the risk of cardiovascular side effects, such as arrhythmia
Hexoprenaline is contraindicated for use with monoamine oxidase inhibitors (MAOIs), tricyclic antidepressant (TCAs), ergot alkaloids, and dihydrotachysterol.[3]
References
- 1 2 "Gynipral (hexoprenaline) Full Prescribing Information". Russian State Register of Medicinal Products (in Russian). Nycomed Austria GmbH. St. Peter-Straße 25, A-4020, Linz, Austria. Retrieved 19 March 2016.
- ↑ Pinder, RM; Brogden, RN; Speight, ™; Avery, GS (July 1977). "Hexoprenaline". Drugs. 14 (1): 1–28. doi:10.2165/00003495-197714010-00001. PMID 195789.
- 1 2 3 "Gynipral (hexoprenaline) Tablets 0.5 mg, Solution for Intravenous Infusion 5 μg/mL (0.0005%)". "RLS" (РЛС): Russian Register of Medical Products (in Russian). Retrieved 19 March 2016.
This article is issued from Wikipedia - version of the 11/27/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.