Allopurinol
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Zyloprim |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682673 |
| Pregnancy category |
|
| Routes of administration | tablet (100, 300 mg), IV |
| ATC code | M04AA01 (WHO) |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 78±20% |
| Protein binding | Negligible |
| Metabolism | hepatic (80% oxypurinol, 10% allopurinol ribosides) |
| Biological half-life | 2 h (oxypurinol 18-30 h) |
| Identifiers | |
| |
| CAS Number |
315-30-0 |
| PubChem (CID) | 2094 |
| IUPHAR/BPS | 6795 |
| DrugBank |
DB00437 |
| ChemSpider |
2010 |
| UNII |
63CZ7GJN5I |
| KEGG |
D00224 |
| ChEBI |
CHEBI:40279 |
| ChEMBL |
CHEMBL1467 |
| Chemical and physical data | |
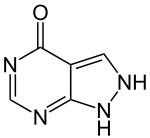
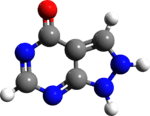
| Formula | C5H4N4O |
| Molar mass | 136.112 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
| (verify) | |
Allopurinol, sold under the brand name Zyloprim and generics, is a medication used primarily to treat excess uric acid in the blood and its complications, including chronic gout.[1] It is a xanthine oxidase inhibitor and is administered orally.
It is on the World Health Organization's List of Essential Medicines, a list of the most important medication needed in a basic health system.[2]
Medical uses
Gout and hyperuricemia
Allopurinol is used to reduce urate/uric acid formation in conditions where urate/uric acid deposition has already occurred or is predictable. The specific diseases and conditions where it used include gouty arthritis, skin tophi, kidney stones idiopathic gout; uric acid lithiasis; acute uric acid nephropathy; neoplastic disease and myeloproliferative disease with high cell turnover rates, in which high urate levels occur either spontaneously, or after cytotoxic therapy; certain enzyme disorders which lead to overproduction of urate, for example: hypoxanthine-guanine phosphoribosyl transferase, including Lesch-Nyhan syndrome; glucose-6-phosphatase including glycogen storage disease; phosphoribosyl pyrophosphate synthetase, phosphoribosyl pyrophosphate amidotransferase; adenine phosphoribosyl transferase.
It is also used to treat kidney stones caused by deficient activity of adenine phosphoribosyltransferase.
Tumor lysis syndrome
Allopurinol was also commonly used to treat tumor lysis syndrome in chemotherapeutic treatments, as these regimens can rapidly produce severe acute hyperuricemia,[3] although it has gradually been replaced by urate oxidase therapy.[4] IV formulations are used in this indication when people cannot take medicine by mouth.[5]
Inflammatory bowel disease
Allopurinol cotherapy is used to improve outcomes for people with inflammatory bowel disease and Crohn's disease who do not respond to thiopurine monotherapy.[6][7] Cotherapy has also been shown to greatly improve hepatoxicity side effects in treatment of IBD.[8] Cotherapy invariably requires dose reduction of the thiopurine, usually to one-third of the standard dose depending upon the patient's genetic status for thiopurine methyltransferase.[9]
Epilepsy
Allopurinol is used as an add-on drug for refractory epilepsy, because it is an adenosine agonist, which inhibits glutamine release from excitatory neurons, but does not change the plasma concentration of other epilepsy drugs.[10]
Contraindications and drug interactions
Allopurinol should not be given to people who are allergic to it.[3]
Drug interactions are extensive, and are as follows:[3]
- Azathioprine and 6-mercaptopurine: Azathioprine is metabolised to 6-mercaptopurine which in turn is inactivated by the action of xanthine oxidase - the target of allopurinol. Giving allopurinol with either of these drugs at their normal dose will lead to overdose of either drug; only one-quarter of the usual dose of 6-mercaptopurine or azathioprine should be given;
- Didanosine: plasma didanosine Cmax and AUC values were approximately doubled with concomitant allopurinol treatment; it should not be co-administered with allopuroinol and if it must be, the dose of should be reduced and the person should be closely monitored.
Allopurinol may also increase the activity or half-life of the following drugs, in order of seriousness and certainty of the interaction:[3]
- Ciclosporin
- Coumarin anticoagulants (reported rarely, but is serious when it occurs)
- Vidarabine
- Chlorpropamide
- Phenytoin
- Theophylline
- Cyclophosphamide, doxorubicin, bleomycin, procarbazine, mechlorethamine
Co-administration of the following drugs may make allopurinol less active or decrease its half-life:[3]
- Salicylates and medicines that increase the secretion of uric acid
- furosemide (see more on diuretics below)/
Co-administration of the following drugs may cause hypersensitivity or skin rash:[3]
- Ampicillin and amoxicillin
- Diuretics, in particular thiazides, especially in renal impairment.
- Angiotensin-converting-enzyme inhibitors (ACE inhibitors)
Side effects
Because allopurinol is not a uricosuric, it can be used in patients with poor kidney function. However, allopurinol has two important disadvantages.
First, its dosing is complex.[11] Second, some patients are hypersensitive to the drug, therefore its use requires careful monitoring.[12][13]
Allopurinol has rare but potentially fatal adverse effects involving the skin. The most serious adverse effect is a hypersensitivity syndrome consisting of fever, skin rash, eosinophilia, hepatitis, and worsened renal function.[12] Allopurinol is one of the drugs commonly known to cause Stevens–Johnson syndrome and toxic epidermal necrolysis, two life-threatening dermatological conditions.[12] More common is a less-serious rash that leads to discontinuing this drug.[12]
More rarely, allopurinol can also result in the depression of bone marrow elements, leading to cytopenias, as well as aplastic anemia. Moreover, allopurinol can also cause peripheral neuritis in some patients, although this is a rare side effect. Another side effect of allopurinol is interstitial nephritis.[14]
Pharmacology
A common misconception is that allopurinol is metabolized by its target, xanthine oxidase, but this action is principally carried out by aldehyde oxidase.[15] The active metabolite of allopurinol is oxypurinol, which is also an inhibitor of xanthine oxidase. Allopurinol is almost completely metabolized to oxypurinol within two hours of oral administration, whereas oxypurinol is slowly excreted by the kidneys over 18–30 hours. For this reason, oxypurinol is believed responsible for the majority of allopurinol's effect.[16]
Mechanism of action
Allopurinol is a purine analog; it is a structural isomer of hypoxanthine (a naturally occurring purine in the body) and is an inhibitor of the enzyme xanthine oxidase.[1] Xanthine oxidase is responsible for the successive oxidation of hypoxanthine and xanthine, resulting in the production of uric acid, the product of human purine metabolism.[1] In addition to blocking uric acid production, inhibition of xanthine oxidase causes an increase in hypoxanthine and xanthine. While xanthine cannot be converted to purine ribotides, hypoxanthine can be salvaged to the purine ribotides adenosine and guanosine monophosphates. Increased levels of these ribotides may cause feedback inhibition of amidophosphoribosyl transferase, the first and rate-limiting enzyme of purine biosynthesis. Allopurinol, therefore, decreases uric acid formation and may also inhibit purine synthesis.[17]
Pharmacogenetics
The HLA-B*5801 allele is a genetic marker for allopurinol-induced severe cutaneous adverse reactions, including Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN).[18][19] The frequency of the HLA-B*5801 allele varies between ethnicities: Han Chinese and Thai populations have HLA-B*5801 allele frequencies of around 8%, as compared to European and Japanese populations, who have allele frequencies of around 1.0% and 0.5%, respectively.[20] The increase in risk for developing allopurinol-induced SJS or TEN in individuals with the HLA-B*5801 allele (as compared to those who do not have this allele) is very high, ranging from a 40-fold to a 580-fold increase in risk, depending on ethnicity.[18][19] As of 2011 the FDA-approved drug label for allopurinol did not contain any information regarding the HLA-B*5801 allele, though FDA scientists did publish a study in 2011 which reported a strong, reproducible and consistent association between the allele and allopurinol-induced SJS and TEN.[21] However, the American College of Rheumatology recommends screening for HLA-B*5801 in high-risk populations (e.g. Koreans with stage 3 or worse chronic kidney disease and those of Han Chinese and Thai descent), and prescribing patients who are positive for the allele an alternative drug.[22] The Clinical Pharmacogenetics Implementation Consortium guidelines state that allopurinol is contraindicated in known carriers of the HLA-B*5801 allele.[23][24]
History
Allopurinol was first synthesized and reported in 1956 by Roland K. Robins (1926-1992), in a search for antineoplastic agents.[1][25] Because allopurinol inhibits the breakdown (catabolism) of the thiopurine drug mercaptopurine, and it was later tested by Wayne Rundles, in collaboration with Gertrude Elion's lab at Wellcome Research Laboratories to see if it could improve treatment of acute lymphoblastic leukemia by enhancing the action of mercaptopurine.[1][26] However, no improvement in leukemia response was noted with mercaptopurine-allopurinol co-therapy, so that work turned to other compounds and the team then started testing allopurinol as a potential for gout.[27] Allopurinol was first marketed as a treatment for gout in 1966.[26]
Society and culture

Formulations
Allopurinol is sold as an injection for intravenous use[5] and as a tablet.[3]
Brands
Allopurinol has been marketed in the United States since August 19, 1966, when it was first approved by FDA under the trade name Zyloprim.[28] Allopurinol was marketed at the time by Burroughs-Wellcome. Allopurinol is now a generic drug sold under a variety of brand names, including Allohexal, Allosig, Milurit, Alloril, Progout, Ürikoliz, Zyloprim, Zyloric, Zyrik, and Aluron.[29]
References
- 1 2 3 4 5 Pacher, P.; Nivorozhkin, A; Szabó, C (2006). "Therapeutic Effects of Xanthine Oxidase Inhibitors: Renaissance Half a Century after the Discovery of Allopurinol". Pharmacological Reviews. 58 (1): 87–114. doi:10.1124/pr.58.1.6. PMC 2233605
 . PMID 16507884.
. PMID 16507884. - ↑ "WHO Model List of EssentialMedicines" (PDF). World Health Organization. October 2013. Retrieved 22 April 2014.
- 1 2 3 4 5 6 7 UK Electronic Medicines Compendium 300 mg Allopurinol tables Last updated April 7, 2016
- ↑ Jeha S. (2001). "Tumor lysis syndrome". Semin Hematol. 38 (4 Suppl 10): 4–8. doi:10.1016/S0037-1963(01)90037-X. PMID 11694945.
- 1 2 FDA Label for injectable Allopurinol Last updated June 2014
- ↑ Bradford K, Shih DQ. Optimizing 6-mercaptopurine and azathioprine therapy in the management of inflammatory bowel disease. World J Gastroenterol. 2011 Oct 7;17(37):4166-73. Review. PMID 22072847 PMC 3208360/
- ↑ Sparrow MP, Hande SA, Friedman S, et al. (2007). "Effect of allopurinol on clinical outcomes in inflammatory bowel disease nonresponders to azathioprine or 6-mercaptopurine". Clin Gastroenterol Hepatol. 5 (2): 209–214. doi:10.1016/j.cgh.2006.11.020. PMID 17296529.
- ↑ Ansari AR, Patel N, Sanderson J, et al. (2010). "Low dose azathioprine or 6-mercaptopurine in combination with allopurinol can bypass many adverse drug reactions in patients with inflammatory bowel disease". Aliment Pharmacol Ther. 31 (6): 640–647. doi:10.1111/j.1365-2036.2009.04221.x. PMID 20015102.
- ↑ Ansari AR, Duley JA (March 2012). "Azathioprine co-therapy with allopurinol for inflammatory bowel disease: trials and tribulations". Rev Assoc Med Bras. 58 (Suppl.1): S28–33.
- ↑ Drug-Resistant Epilepsy N Engl J Med 2011; 365:2238-2240December 8, 2011
- ↑ Dalbeth, Nicola; Stamp, Lisa (2007). "Allopurinol Dosing in Renal Impairment: Walking the Tightrope Between Adequate Urate Lowering and Adverse Events". Seminars in Dialysis. 20 (5): 391–5. doi:10.1111/j.1525-139X.2007.00270.x. PMID 17897242.
- 1 2 3 4 Chung, WH; Wang, CW; Dao, RL (July 2016). "Severe cutaneous adverse drug reactions.". The Journal of dermatology. 43 (7): 758–66. PMID 27154258.
- ↑ Tsai TF, Yeh TY (2010). "Allopurinol in dermatology". Am J Clin Dermatol. 11 (4): 225–232. doi:10.2165/11533190-000000000-00000. PMID 20509717.
- ↑ Marc E. De Broe; William M. Bennett; George A. Porter (2003). Clinical Nephrotoxins: Renal Injury from Drugs and Chemicals. Springer Science+Business Media. ISBN 9781402012778.
Acute interstitial nephritis has also been reported associated with by the administration of allopurinol.
- ↑ Reiter S, Simmonds HA, Zöllner N, et al. (1990). "Demonstration of a combined deficiency of xanthine oxidase and aldehyde oxidase in xanthinuric patients not forming oxipurinol". Clin Chim Acta. 187 (3): 221–234. doi:10.1016/0009-8981(90)90107-4. PMID 2323062.
- ↑ Day RO, Graham GG, Hicks M, et al. (2007). "Clinical pharmacokinetics and pharmacodynamics of allopurinol and oxypurinol". Clin Pharmacokinet. 46 (8): 623–644. doi:10.2165/00003088-200746080-00001. PMID 17655371.
- ↑ Cameron JS, Moro F, Simmonds HA (1993). "Gout, uric acid and purine metabolism in paediatric nephrology". Pediatr Nephrol. 7 (1): 105–118. doi:10.1007/BF00861588. PMID 8439471.
- 1 2 http://www.pharmgkb.org/haplotype/PA165956630#tabview=tab3&subtab=
- 1 2 http://www.pharmgkb.org/pathway/PA165980774
- ↑ http://www.allelefrequencies.net
- ↑ Zineh I, Mummaneni P, Lyndly J, et al. (December 2011). "Allopurinol pharmacogenetics: assessment of potential clinical usefulness". Pharmacogenomics. 12 (12): 1741–9. doi:10.2217/pgs.11.131. PMID 22118056.
- ↑ Khanna D, Fitzgerald JD, Khanna PP, et al. (October 2012). "2012 American College of Rheumatology guidelines for management of gout. Part 1: systematic nonpharmacologic and pharmacologic therapeutic approaches to hyperuricemia". Arthritis Care Res (Hoboken). 64 (10): 1431–46. doi:10.1002/acr.21772. PMC 3683400
 . PMID 23024028.
. PMID 23024028. - ↑ http://www.pharmgkb.org/guideline/PA166105003
- ↑ Hershfield MS, Callaghan JT, Tassaneeyakul W, et al. (February 2013). "Clinical Pharmacogenetics Implementation Consortium guidelines for human leukocyte antigen-B genotype and allopurinol dosing". Clin Pharmacol Ther. 93 (2): 153–8. doi:10.1038/clpt.2012.209. PMC 3564416
 . PMID 23232549.
. PMID 23232549. - ↑ R. K. Robins (1956). "Potential Purine Antagonists. I. Synthesis of Some 4,6-Substituted Pyrazolo \3,4-d] pyrimidines1". J. Amer. Chem. Soc. 78 (4): 784. doi:10.1021/ja01585a023.
- 1 2 Walter Sneader. Drug Discovery: A History. John Wiley & Sons, 2005 ISBN 9780471899792. page 254
- ↑ Elion GB. (1989). "The purine path to chemotherapy (Nobel lecture in physiology or medicine - 1988)". Science. 244 (4900): 41–47. doi:10.1126/science.2649979. PMID 2649979.
- ↑ http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm
- ↑ http://dailymed.nlm.nih.gov/dailymed/search.cfm?startswith=Allopurinol&x=0&y=0
Further reading
- Zahran AM, Azab KS, Abbady MI (2006). "Modulatory role of allopurinol on xanthine oxidoreductase system and antioxidant status in irradiated rats". Egyptian Journal of Radiation Sciences and Applications. 19 (2): 373–388. ISSN 1110-0303.
- Hung, Shuen-Iu; Chung, Wen-Hung; Liou, Lieh-Bang; Chu, Chen-Chung; Lin, Marie; Huang, Hsien-Ping; Lin, Yen-Ling; Lan, Joung-Liang; Yang, Li-Cheng; Hong, H.-S.; Chen, M.-J.; Lai, P.-C.; Wu, M.-S.; Chu, C.-Y.; Wang, K.-H.; Chen, C.-H.; Fann, C. S. J.; Wu, J.-Y.; Chen, Y.-T. (2005). "HLA-B*5801 allele as a genetic marker for severe cutaneous adverse reactions caused by allopurinol". Proceedings of the National Academy of Sciences. 102 (11): 4134–9. doi:10.1073/pnas.0409500102. PMC 554812
 . PMID 15743917.
. PMID 15743917.
- The Third International Thiopurine Symposium 2010, published in RAMB, for information on Allopurinol co-therapy:
External links
- Zyloprim (patient information)
- Allopurinol pathway on PharmGKB
- Very Important Pharmacogene summary for HLA-B on PharmGKB
- Understanding Allopurinol (side effects)