Regadenoson
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | Multum Consumer Information |
| License data |
|
| Pregnancy category |
|
| Routes of administration | Intravenous |
| ATC code | C01EB21 (WHO) |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
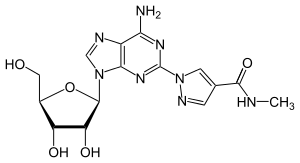
| Synonyms | 1-[6-amino-9-[(2R,3R,4S,5R)-3,4-dihydroxy-5-(hydroxymethyl)oxolan-2-yl]purin-2-yl]- N-methylpyrazole-4-carboxamide |
| CAS Number |
313348-27-5 |
| PubChem (CID) | 219024 |
| IUPHAR/BPS | 5596 |
| DrugBank |
DB06213 |
| ChemSpider |
189859 |
| UNII |
7AXV542LZ4 |
| KEGG |
D05711 |
| ChEMBL |
CHEMBL1201750 |
| ECHA InfoCard | 100.218.278 |
| Chemical and physical data | |
| Formula | C15H18N8O5 |
| Molar mass | 390.354 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
| | |
Regadenoson (CVT-3146, Lexiscan) is an A2A adenosine receptor agonist that is a coronary vasodilator that is commonly used in pharmacologic stress testing. It produces hyperemia quickly and maintains it for a duration that is useful for radionuclide myocardial perfusion imaging.[1] The selective nature of the drug makes it preferable to other stress agents such as adenosine, which are less selective and therefore cause more side-effects.
Regadenoson was approved by the United States Food and Drug Administration on April 10, 2008 and is marketed by Astellas Pharma under the tradename Lexiscan.[2] It is approved for use in the European Union and under the name of Rapiscan. It is currently being marketed by GE Healthcare and is being sold in both the United Kingdom and Germany.
Regadenoson has a 2 to 3 minute biological half-life, as compared with adenosine's 10-second half-life. As a result, regadenoson stress protocols use a single bolus, instead of a 4-6 minute continuous infusion, which was needed with adenosine. Another difference is that adenosine infusion is weight based (140mcg/kg/minute), while with regadenoson, a 0.4mg/5mL preloaded syringe dose is standard for all weights. Regadenoson stress tests are not affected by the presence of beta blockers, as regadenoson vasodilates via the adenosine pathway without stimulating beta adrenergic receptors.
One side effect of regadenoson is that it can temporarily disrupt the integrity of the blood-brain barrier by inhibiting P-glycoprotein function.[3]
References
- ↑ Cerqueira MD (July 2004). "The future of pharmacologic stress: selective A2A adenosine receptor agonists". Am. J. Cardiol. 94 (2A): 33D–40D; discussion 40D–42D. doi:10.1016/j.amjcard.2004.04.017. PMID 15261132.
- ↑ CV Therapeutics and Astellas Announce FDA Approval for Lexiscan(TM)
- ↑ Do-Geun Kim, Margaret S. Bynoe. A2A adenosine receptor modulates drug efflux transporter P-glycoprotein at the blood-brain barrier. Journal of Clinical Investigation, 2016; doi: 10.1172/JCI76207