Phosphofructokinase 2
| 6-phosphofructo-2-kinase | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||
| EC number | 2.7.1.105 | ||||||||
| CAS number | 78689-77-7 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| Gene Ontology | AmiGO / EGO | ||||||||
| |||||||||
| fructose-2,6-bisphosphate 2-phosphatase | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||
| EC number | 3.1.3.46 | ||||||||
| CAS number | 81611-75-8 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| Gene Ontology | AmiGO / EGO | ||||||||
| |||||||||
| 6-phosphofructo-2-kinase | |||||||||
|---|---|---|---|---|---|---|---|---|---|
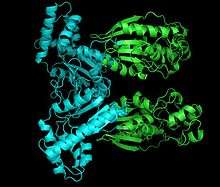 Structure of PFK2. Shown: kinase domain (cyan) and the phosphatase domain (green). | |||||||||
| Identifiers | |||||||||
| Symbol | 6PF2K | ||||||||
| Pfam | PF01591 | ||||||||
| InterPro | IPR013079 | ||||||||
| PROSITE | PDOC00158 | ||||||||
| SCOP | 1bif | ||||||||
| SUPERFAMILY | 1bif | ||||||||
| |||||||||
Phosphofructokinase 2 (PFK2) or fructose bisphosphatase 2 (FBPase2), is an enzyme responsible for regulating the rates of glycolysis and gluconeogenesis in the human body. It is a homodimer of 55 kDa subunits arranged in a head-to-head fashion, with each polypeptide chain consisting of independent kinase and phosphatase domain. When Ser-32 of the bifunctional protein is phosphorylated, the negative charge causes the conformation change of the enzyme to favor the FBPase2 activity; otherwise, PFK2 activity is favored.[1] The PFK2 domain is closely related to the superfamily of mononucleotide binding proteins including adenylate cyclase, whereas that of FBPase2 is related to a family of proteins that include phosphoglycerate mutases.
Structure
The monomers of the bifunctional protein are clearly divided into two functional domains. The kinase domain is located on the N-terminal.[2] It consists of a central six-stranded β sheet, with five parallel strands and an antiparallel edge strand, surrounded by seven α helices.[3] The domain contains nucleotide-binding fold (nbf) at the C-terminal end of the first β-strand,[4] and thus resembles the structure of adenylate kinase.
On the other hand, the phosphatase domain is located on the C-terminal.[5] It resembles the family of proteins that include phosphoglycerate mutases (PGMs) and acid phosphatases.[6] The domain has a mixed α/ β structure, with a six-stranded central β sheet, plus an additional α-helical subdomain that covers the presumed active site of the molecule.[3] Finally, N-terminal region modulates PFK2 and FBPase2 activities, and stabilizes the dimer form of the enzyme.[6][7]
Function
When glucose level is low, glucagon is released into the bloodstream, triggering a cAMP signal cascade. In the liver Protein kinase A inactivates the PFK-2 domain of the bifunctional enzyme via phosphorylation, however this does not occur in skeletal muscle. The F-2,6-BPase domain is then activated which lowers fructose 2,6-bisphosphate (F-2,6-BP) levels. Because F-2,6-BP normally stimulates phosphofructokinase-1(PFK1), the decrease in its concentration leads to the inhibition of glycolysis and the stimulation of gluconeogenesis.[8]
On the other hand, when the glucose level increases, the level of fructose 6-phosphate (F6P) subsequently rises and the molecule stimulates phosphoprotein phosphatase-1, which removes phosphoryl group from the bifunctional protein. So PFK2 domain is activated and the kinase catalyzes the formation of F-2,6-BP. Thus, glycolysis is stimulated and gluconeogenesis is inhibited.
Regulation
The allosteric regulation of PFK2 is very similar to the regulation of PFK1.[9] High levels of AMP or phosphate group signifies a low energy state and thus stimulates PFK2. On the other hand, a high concentration of phosphoenolpyruvate(PEP) and citrate signifies that there is a high level of biosynthetic precursor and hence inhibits PFK2. However, unlike PFK1, PFK2 is not affected by the ATP concentration.
Glucagon inhibits PFK2 by activating Protein Kinase A (PKA), which phosphorylates the PFK2 complex and causes its FBPase activity to be favored; via PKA and PFK2/FBP, glucagon decreases [F-2,6-BP], which inhibits glycolysis by allosteric inhibition of PFK1. Insulin activates PFK2 by activating protein phosphatase, which dephosphorylates the PFK-2 complex and causes its PFK2 activity to be favored; via Protein Phosphatase and PFK2, insulin increases [F-2,6-BP], which activates glycolysis by allosteric activation of PFK1, signalling an abundance of glucose
Reaction mechanism
PFK2 is likely to catalyze the "simple" transfer of γ-phosphoryl group of ATP onto the hydroxyl present on C-2 of fructose-6-phosphate. Yet, the formation of fructose 2,6-bisphosphate could theoretically occur by a variety of mechanisms, including the intermediary formation of Fructose-6-phosphate 2-pyrophosphate.[9]
The hydrolysis of fructose 2,6-biphosphate is likely to follow the below steps:[10]
- Histidine acts as a nucleophile and attacks the 2-phosphate of F-2,6-BP
- The stabilization of pentacoordinated transition state by several salt bridges and hydrogen bonding.
- The breakdown of the transition state and the release of F6P.
- Histidine increases the nucleophilicity of water, which attacks phosphohistidine, generating phosphate and newly protonated histidine.
Clinical significance
The Pfkfb2 gene encoding PFK2/FBPase2 protein is linked to the predisposition to schizophrenia.[11] Furthermore, the control of PFK2/FBPase2 activity was found to be linked to heart functioning and the control against hypoxia.[12]
Isozymes
Five mammalian isozymes of the protein have been reported to date, difference rising by either the transcription of different enzymes or alternative splicing.[13][14][15]The isozymes differ radically in their regulation and the discussions above are based on liver isozyme.[3]
Humans genes encoding proteins possessing phosphofructokinase 2 activity include:
References
- ↑ Kurland IJ, el-Maghrabi MR, Correia JJ, Pilkis SJ (March 1992). "Rat liver 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase. Properties of phospho- and dephospho- forms and of two mutants in which Ser32 has been changed by site-directed mutagenesis". J. Biol. Chem. 267 (7): 4416–23. PMID 1339450.
- ↑ Kurland I, Chapman B, Lee YH, Pilkis S (August 1995). "Evolutionary reengineering of the phosphofructokinase active site: ARG-104 does not stabilize the transition state in 6-phosphofructo-2-kinase". Biochem. Biophys. Res. Commun. 213 (2): 663–72. doi:10.1006/bbrc.1995.2183. PMID 7646523.
- 1 2 3 Hasemann CA, Istvan ES, Uyeda K, Deisenhofer J (September 1996). "The crystal structure of the bifunctional enzyme 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase reveals distinct domain homologies". Structure. 4 (9): 1017–29. doi:10.1016/S0969-2126(96)00109-8. PMID 8805587.
- ↑ Walker JE, Saraste M, Runswick MJ, Gay NJ (1982). "Distantly related sequences in the alpha- and beta-subunits of ATP synthase, myosin, kinases and other ATP-requiring enzymes and a common nucleotide binding fold". EMBO J. 1 (8): 945–51. PMC 553140
 . PMID 6329717.
. PMID 6329717. - ↑ Li L, Lin K, Pilkis J, Correia JJ, Pilkis SJ (October 1992). "Hepatic 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase. The role of surface loop basic residues in substrate binding to the fructose-2,6-bisphosphatase domain". J. Biol. Chem. 267 (30): 21588–94. PMID 1328239.
- 1 2 Stryer, Lubert; Berg, Jeremy Mark; Tymoczko, John L. (2008). "The Balance Between Glycolysis and Gluconeogenesis in the Liver Is Sensitive to Blood-Glucose Concentration". Biochemistry (Looseleaf). San Francisco: W. H. Freeman. pp. 466–467. ISBN 1-4292-3502-0.
- ↑ Tominaga N, Minami Y, Sakakibara R, Uyeda K (July 1993). "Significance of the amino terminus of rat testis fructose-6-phosphate, 2-kinase:fructose-2,6-bisphosphatase". J. Biol. Chem. 268 (21): 15951–7. PMID 8393455.
- ↑ Pilkis SJ, Claus TH, Kurland IJ, Lange AJ (1995). "6-Phosphofructo-2-kinase/fructose-2,6-bisphosphatase: a metabolic signaling enzyme". Annu. Rev. Biochem. 64: 799–835. doi:10.1146/annurev.bi.64.070195.004055. PMID 7574501.
- 1 2 Van Schaftingen E, Hers HG (August 1981). "Phosphofructokinase 2: the enzyme that forms fructose 2,6-bisphosphate from fructose 6-phosphate and ATP". Biochem. Biophys. Res. Commun. 101 (3): 1078–84. doi:10.1016/0006-291X(81)91859-3. PMID 6458291.
- ↑ Lin K, Li L, Correia JJ, Pilkis SJ (April 1992). "Glu327 is part of a catalytic triad in rat liver fructose-2,6-bisphosphatase". J. Biol. Chem. 267 (10): 6556–62. PMID 1313012.
- ↑ Stone WS, Faraone SV, Su J, Tarbox SI, Van Eerdewegh P, Tsuang MT (May 2004). "Evidence for linkage between regulatory enzymes in glycolysis and schizophrenia in a multiplex sample". Am. J. Med. Genet. B Neuropsychiatr. Genet. 127B (1): 5–10. doi:10.1002/ajmg.b.20132. PMID 15108172.
- ↑ Wang Q, Donthi RV, Wang J, Lange AJ, Watson LJ, Jones SP, Epstein PN (June 2008). "Cardiac phosphatase-deficient 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase increases glycolysis, hypertrophy, and myocyte resistance to hypoxia". Am. J. Physiol. Heart Circ. Physiol. 294 (6): H2889–97. doi:10.1152/ajpheart.91501.2007. PMID 18456722.
- ↑ Darville MI, Crepin KM, Hue L, Rousseau GG (September 1989). "5' flanking sequence and structure of a gene encoding rat 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase". Proc. Natl. Acad. Sci. U.S.A. 86 (17): 6543–7. doi:10.1073/pnas.86.17.6543. PMC 297880
 . PMID 2549541.
. PMID 2549541. - ↑ Tsuchiya Y, Uyeda K (May 1994). "Bovine heart fructose 6-P,2-kinase:fructose 2,6-bisphosphatase mRNA and gene structure". Arch. Biochem. Biophys. 310 (2): 467–74. doi:10.1006/abbi.1994.1194. PMID 8179334.
- ↑ Sakata J, Abe Y, Uyeda K (August 1991). "Molecular cloning of the DNA and expression and characterization of rat testes fructose-6-phosphate,2-kinase:fructose-2,6-bisphosphatase". J. Biol. Chem. 266 (24): 15764–70. PMID 1651918.
Further reading
- Rider MH, Bertrand L, Vertommen D, Michels PA, Rousseau GG, Hue L (August 2004). "6-Phosphofructo-2-kinase/fructose-2,6-bisphosphatase: head-to-head with a bifunctional enzyme that controls glycolysis". Biochem. J. 381 (Pt 3): 561–79. doi:10.1042/BJ20040752. PMC 1133864
 . PMID 15170386.
. PMID 15170386.
External links
- Fructose 2,6-bisphosphatase at the US National Library of Medicine Medical Subject Headings (MeSH)
- 6-phosphofructokinase of Arabidopsis thaliana at genome.jp