IDFP
 | |
| Names | |
|---|---|
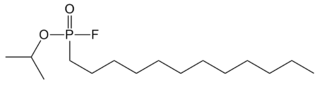
| IUPAC name
isopropyl dodecylphosphonofluoridate | |
| Identifiers | |
| 615250-02-7 | |
| 3D model (Jmol) | Interactive image |
| ChemSpider | 21169067 |
| PubChem | 24762154 |
| |
| |
| Properties | |
| C15H32FO2P | |
| Molar mass | 294.39 g/mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
IDFP is an organophosphorus compound related to the nerve agent sarin. Like sarin, IDFP is an irreversible inhibitor for a number of different enzymes that normally serve to break down neurotransmitters, however the long alkyl chain of IDFP makes it dramatically weaker as an inhibitor of acetylcholinesterase (AChE), with an IC50 of only 6300nM, while it is a potent inhibitor of two enzymes monoacylglycerol lipase (MAGL), the primary enzyme responsible for degrading the endocannabinoid 2-arachidonoylglycerol (2-AG), and fatty acid amide hydrolase (FAAH), the primary enzyme that degrades the other main endocannabinoid anandamide. The IC50 of IDFP is 0.8nM at MAGL, and 3.0nM at FAAH. Inhibition of these two enzymes causes markedly increased levels of both anandamide and 2-AG in the brain, resulting in increased cannabinoid signalling and typical cannabinoid behavioral effects in animal studies, while its lack of potency at AChE means that no cholinergic symptoms are produced.[1][2][3][4] Despite its similar chemical structure to the banned nerve agents, the long alkyl chain of IDFP causes it to fall outside the definition of "toxic chemicals" under the Chemical Weapons Convention,[5] and since it also does not exhibit the potent AChE inhibition of related organophosphorus compounds, IDFP is not subject to the same stringent legal controls.
See also
References
- ↑ Nomura, D. K.; Blankman, J. L.; Simon, G. M.; Fujioka, K.; Issa, R. S.; Ward, A. M.; Cravatt, B. F.; Casida, J. E. (2008). "Activation of the endocannabinoid system by organophosphorus nerve agents". Nature Chemical Biology. 4 (6): 373–378. doi:10.1038/nchembio.86. PMC 2597283
 . PMID 18438404.
. PMID 18438404. - ↑ Casida, J. E.; Nomura, D. K.; Vose, S. C.; Fujioka, K. (2008). "Organophosphate-sensitive lipases modulate brain lysophospholipids, ether lipids and endocannabinoids". Chemico-Biological Interactions. 175 (1–3): 355–364. doi:10.1016/j.cbi.2008.04.008. PMC 2582404
 . PMID 18495101.
. PMID 18495101. - ↑ Ruby, M. A.; Nomura, D. K.; Hudak, C. S. S.; Mangravite, L. M.; Chiu, S.; Casida, J. E.; Krauss, R. M. (2008). "Overactive endocannabinoid signaling impairs apolipoprotein E-mediated clearance of triglyceride-rich lipoproteins". Proceedings of the National Academy of Sciences. 105 (38): 14561–14566. doi:10.1073/pnas.0807232105. PMC 2567196
 . PMID 18794527.
. PMID 18794527. - ↑ Ruby, M. A.; Nomura, D. K.; Hudak, C. S. S.; Barber, A.; Casida, J. E.; Krauss, R. M. (2011). Bartolomucci, Alessandro, ed. "Acute Overactive Endocannabinoid Signaling Induces Glucose Intolerance, Hepatic Steatosis, and Novel Cannabinoid Receptor 1 Responsive Genes". PLoS ONE. 6 (11): e26415. doi:10.1371/journal.pone.0026415. PMC 3208546
 . PMID 22073164.
. PMID 22073164. - ↑ CWC Schedule 1 Part A. Toxic Chemicals