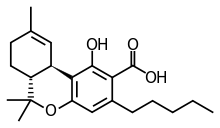
Tetrahydrocannabinolic acid
 | |
 | |
| Clinical data | |
|---|---|
| ATC code | none |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | 23978-85-0 |
| PubChem (CID) | 98523 |
| ChemSpider | 88974 |
| ECHA InfoCard | 100.216.805 |
| Chemical and physical data | |
| Formula | C22H30O4 |
| Molar mass | 358.4733 g/mol |
| 3D model (Jmol) | Interactive image |
| |

Tetrahydrocannabinolic acid (THCA, Δ9-THCA, 2-COOH-THC; conjugate base tetrahydrocannabinolate) is a biosynthetic precursor of tetrahydrocannabinol (THC), the active component of cannabis.[1][2][3][4] When purified, it forms a powder which is unstable in the presence of acids, heat, oxygen, and/or light.[5][6]
THCA is found in variable quantities in fresh, undried cannabis, but is progressively decarboxylated to THC with drying, and especially under intense heating such as when cannabis is smoked[7] or cooked into cannabis edibles.
Pharmacological effects
THCA does not have any known psychoactive effects on humans in its own right.[8] It does have antiinflammatory,[9] neuroprotective,[10] antiemetic (anti-vomitting)[11] and anti-prostate cancer effects.[12] It inhibits COX-1 and COX-2 enzymes involved in inflammation in human colon cell cultures.[9] It has also been shown to decrease the amount of oxidative stress caused by impairment of mitochondria which is a major mechanism in neural degeneration in mouse mesencephalic cell cultures.[10]
Uses
Despite the ready decarboxylation by drying or heating ex vivo, conversion of THCA to THC in vivo appears to be very limited, giving it only very slight efficacy as a prodrug for THC.[13] Consequently, it is believed to be important in less-psychoactive preparations of cannabis used for medical use, such as cannabis tea.[14]
THCA is commonly used as a biomarker in drug testing along with THCV, to distinguish between prescribed synthetic Delta-9-tetrahydrocannabinol, such as Marinol, and cannabis plant material which may also be used by patients.[15]
Legal status
THCA is not scheduled by the United Nations' Convention on Psychotropic Substances.[16]
United States
THCA is not scheduled at the federal level in the United States,[17] but it is possible that THCA could legally be considered an analog of THC and sales or possession could potentially be prosecuted under the Federal Analogue Act.[18]
There is also a practical legal issue of potential THC contamination of THCA which should be considered. As mentioned above, THCA decarboxylates to form THC, a Schedule I controlled substance. As such it is somewhat unlikely that any sample of THCA which may be analytically tested will not contain a quantifiable amount of THC, especially if the sampled product was retained at temperatures conducive to decarboxylation. This makes it likely that possession of THCA could get someone prosecuted for possession of THC due to contamination of the THCA with THC.
See also
References
- ↑ Baker PB, Taylor BJ, Gough TA (Jun 1981), "The tetrahydrocannabinol and tetrahydrocannabinolic acid content of cannabis products", Journal of Pharmacy and Pharmacology, 33 (6): 369–72, doi:10.1111/j.2042-7158.1981.tb13806.x, PMID 6115009
- ↑ Sirikantaramas S, Morimoto S, Shoyama Y, Ishikawa Y, Wada Y, Shoyama Y, Taura F (2004-09-17), "The gene controlling marijuana psychoactivity: molecular cloning and heterologous expression of Delta1-tetrahydrocannabinolic acid synthase from Cannabis sativa L.", Journal of Biological Chemistry, 279 (38): 39767–74, doi:10.1074/jbc.M403693200, PMID 15190053
- ↑ Moore C, Rana S, Coulter C (2007-06-01), "Simultaneous identification of 2-carboxy-tetrahydrocannabinol, tetrahydrocannabinol, cannabinol and cannabidiol in oral fluid", J Chromatogr B Analyt Technol Biomed Life Sci., 852 (1-2): 459–64, doi:10.1016/j.jchromb.2007.02.016, PMID 17321807
- ↑ Taura F. (Jun 2009), "Studies on tetrahydrocannabinolic acid synthase that produces the acidic precursor of tetrahydrocannabinol, the pharmacologically active cannabinoid in marijuana", Drug Discoveries and Therapeutics, 3 (3): 83–7, PMID 22495534
- ↑ Δ9-Tetrahydrocannabinolic Acid (Δ9-THCA) @ Aphios.com
- ↑ Zoller O, Rhyn P, Zimmerli B ("2000"), "High-performance liquid chromatographic determination of Δ9-tetrahydrocannabinol and the corresponding acid in hemp containing foods with special regard to the fluorescence properties of Δ9–tetrahydrocannabinol", Journal of Chromatography A, 872: 101–110, doi:10.1016/s0021-9673(99)01287-x Check date values in:
|date=(help) - ↑ Dussy FE, Hamberg C, Luginbühl M, Schwerzmann T, Briellmann TA (2005-04-20), "Isolation of Delta9-THCA-A from hemp and analytical aspects concerning the determination of Delta9-THC in cannabis products", Forensic Science International, 149 (1): 3–10, doi:10.1016/j.forsciint.2004.05.015, PMID 15734104
- ↑ Starks, Michael (1990). Marijuana Chemistry: Genetics, Processing, Potency. Ronin Publishing. ISBN 978-0-9141-7139-3.
- 1 2 Ruhaak LR, Felth J, Karlsson PC, Rafter JJ, Verpoorte R, Bohlin L (2011), "Evaluation of the cyclooxygenase inhibiting effects of six major cannabinoids isolated from Cannabis sativa", Biological and Pharmaceutical Bulletin, 34 (5): 774–8, doi:10.1248/bpb.34.774, PMID 21532172
- 1 2 Moldzio R, Pacher T, Krewenka C, Kranner B, Novak J, Duvigneau JC, Rausch WD (2012-05-07), "Effects of cannabinoids Δ(9)-tetrahydrocannabinol, Δ(9)-tetrahydrocannabinolic acid and cannabidiol in MPP(+) affected murine mesencephalic cultures", Phytomedicine, 19 (8-9): 819–24, doi:10.1016/j.phymed.2012.04.002, PMID 22571976
- ↑ Rock EM, Kopstick RL, Limebeer CL, Parker LA (2013). "Tetrahydrocannabinolic acid reduces nausea-induced conditioned gaping in rats and vomiting in Suncus murinus". Br. J. Pharmacol. 170: 641–8. doi:10.1111/bph.12316. PMC 3792001
 . PMID 23889598.
. PMID 23889598. - ↑ De Petrocellis L; Ligresti A.; Moriello A.S.; Iappelli M.; Verde R.; Stott C.G.; Cristino L.; Orlando P. & Di Marzo V. (2013-01-01), "Non-THC cannabinoids inhibit prostate carcinoma growth in vitro and in vivo: pro-apoptotic effects and underlying mechanisms", British Journal of Pharmacology, 168 (1): 79–102, doi:10.1111/j.1476-5381.2012.02027.x, PMC 3570006
 , PMID 22594963
, PMID 22594963 - ↑ Jung J, Meyer MR, Maurer HH, Neusüss C, Weinmann W, Auwärter V (Oct 2009), "Studies on the metabolism of the Delta-9-tetrahydrocannabinol precursor delta-9-tetrahydrocannabinolic acid A (Delta9-THCA-A) in rat using LC-MS/MS, LC-QTOF MS and GC-MS techniques", Journal of Mass Spectrometry, 44 (10): 1423–33, doi:10.1002/jms.1624, PMID 19728318
- ↑ Hazekamp A, Bastola K, Rashidi H, Bender J, Verpoorte R (2007-07-15), "Cannabis tea revisited: a systematic evaluation of the cannabinoid composition of cannabis tea", Journal of Ethnopharmacology, 113 (1): 85–90, doi:10.1016/j.jep.2007.05.019, PMID 17604926
- ↑ Radünz L, Westphal F, Maser E, Rochholz G (2012-02-10), "THCVA-A - a new additional marker for illegal cannabis consumption", Forensic Science International, 215 (1-3): 171–4, doi:10.1016/j.forsciint.2011.03.001, PMID 21454026
- ↑ Convention on Psychotropic Substances, 1971
- ↑ §1308.11 Schedule I.
- ↑ Erowid Analog Law Vault : Federal Controlled Substance Analogue Act Summary