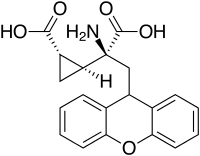
LY-341,495
 | |
| Identifiers | |
|---|---|
| |
| Synonyms | (2S)-2-Amino-2-[(1S,2S)-2-carboxycycloprop-1-yl]-3-(xanth-9-yl)propanoic acid |
| CAS Number |
201943-63-7 |
| ChemSpider |
7995676 |
| Chemical and physical data | |
| Formula | C20H19NO5 |
| Molar mass | 353.37 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
| | |
LY-341,495 is a research drug developed by the pharmaceutical company Eli Lilly, which acts as a potent and selective orthosteric antagonist for the group II metabotropic glutamate receptors (mGluR2/3).[1][2][3][4]
It is used in scientific research in several different areas, showing antidepressant effects in animal models,[5][6][7] increasing the behavioural effects of hallucinogenic drugs in animal tests,[8][9][10] and increasing the analgesic effects of μ-opioid agonists,[11][12] as well as modulating dopamine receptor function.[13][14][15]
The 1-fluorocyclopropane analog has a superior pharmacokinetic profile and similar mGluR2/3 affinity, and making a prodrug from this with the heptyl ester increases bioavailability still further.[16]
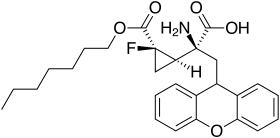
1-fluoro-LY-341,495 heptyl ester
See also
References
- ↑ Ornstein PL, Bleisch TJ, Arnold MB, Kennedy JH, Wright RA, Johnson BG, Tizzano JP, Helton DR, Kallman MJ, Schoepp DD, Hérin M. 2-substituted (2SR)-2-amino-2-((1SR,2SR)-2-carboxycycloprop-1-yl)glycines as potent and selective antagonists of group II metabotropic glutamate receptors. 2. Effects of aromatic substitution, pharmacological characterization, and bioavailability. Journal of Medicinal Chemistry. 1998 Jan 29;41(3):358-78. PMID 9464367.
- ↑ Kingston AE, Ornstein PL, Wright RA, Johnson BG, Mayne NG, Burnett JP, Belagaje R, Wu S, Schoepp DD. LY341495 is a nanomolar potent and selective antagonist of group II metabotropic glutamate receptors. Neuropharmacology. 1998;37(1):1-12. PMID 9680254 .
- ↑ Fitzjohn SM, Bortolotto ZA, Palmer MJ, Doherty AJ, Ornstein PL, Schoepp DD, Kingston AE, Lodge D, Collingridge GL. The potent mGlu receptor antagonist LY341495 identifies roles for both cloned and novel mGlu receptors in hippocampal synaptic plasticity. Neuropharmacology. 1998 Dec;37(12):1445-58. PMID 9886667
- ↑ Johnson BG, Wright RA, Arnold MB, Wheeler WJ, Ornstein PL, Schoepp DD. [3H]-LY341495 as a novel antagonist radioligand for group II metabotropic glutamate (mGlu) receptors: characterization of binding to membranes of mGlu receptor subtype expressing cells. Neuropharmacology. 1999 Oct;38(10):1519-29. PMID 10530814.
- ↑ Pilc A, Chaki S, Nowak G, Witkin JM. Mood disorders: regulation by metabotropic glutamate receptors. Biochemical Pharmacology. 2008 Mar 1;75(5):997-1006. PMID 18164691
- ↑ Matrisciano F, Panaccione I, Zusso M, Giusti P, Tatarelli R, Iacovelli L, Mathé AA, Gruber SH, Nicoletti F, Girardi P. Group-II metabotropic glutamate receptor ligands as adjunctive drugs in the treatment of depression: a new strategy to shorten the latency of antidepressant medication? Molecular Psychiatry. 2007 Aug;12(8):704-6. PMID 17653204
- ↑ Koike H, Chaki S. Requirement of AMPA receptor stimulation for the sustained antidepressant activity of ketamine and LY341495 during the forced swim test in rats. Behavioral Brain Research. 2014 Sep 1;271:111-5. doi: 10.1016/j.bbr.2014.05.065. PMID 24909673
- ↑ Gewirtz JC, Marek GJ. Behavioral evidence for interactions between a hallucinogenic drug and group II metabotropic glutamate receptors. Neuropsychopharmacology. 2000 Nov;23(5):569-76. PMID 11027922
- ↑ Benneyworth MA, Xiang Z, Smith RL, Garcia EE, Conn PJ, Sanders-Bush E. A selective positive allosteric modulator of metabotropic glutamate receptor subtype 2 blocks a hallucinogenic drug model of psychosis. Molecular Pharmacology. 2007 Aug;72(2):477-84. PMID 17526600
- ↑ Carbonaro TM, Eshleman AJ, Forster MJ, Cheng K, Rice KC, Gatch MB. The role of 5-HT2A, 5-HT 2C and mGlu2 receptors in the behavioral effects of tryptamine hallucinogens N,N-dimethyltryptamine and N,N-diisopropyltryptamine in rats and mice. Psychopharmacology (Berlin). 2014 Jul 3. PMID 24985890
- ↑ Fischer BD, Miller LL, Henry FE, Picker MJ, Dykstra LA. Increased efficacy of mu-opioid agonist-induced antinociception by metabotropic glutamate receptor antagonists in C57BL/6 mice: comparison with (−)-6-phosphonomethyl-deca-hydroisoquinoline-3-carboxylic acid (LY235959). Psychopharmacology (Berlin). 2008 Apr 8; PMID 18392754
- ↑ Fischer BD, Zimmerman EI, Picker MJ, Dykstra LA. Morphine in combination with metabotropic glutamate receptor antagonists on schedule-controlled responding and thermal nociception. Journal of Pharmacology and Experimental Therapeutics. 2008 Feb;324(2):732-9. PMID 17982001
- ↑ O'Neill MF, Heron-Maxwell C, Conway MW, Monn JA, Ornstein P (October 2003). "Group II metabotropic glutamate receptor antagonists LY341495 and LY366457 increase locomotor activity in mice". Neuropharmacology. 45 (5): 565–74. doi:10.1016/S0028-3908(03)00232-6. PMID 12941370.
- ↑ Chi H, Jang JK, Kim JH, Vezina P (October 2006). "Blockade of group II metabotropic glutamate receptors in the nucleus accumbens produces hyperlocomotion in rats previously exposed to amphetamine". Neuropharmacology. 51 (5): 986–92. doi:10.1016/j.neuropharm.2006.06.008. PMID 16901517.
- ↑ Yoon HS, Jang JK, Kim JH (September 2008). "Blockade of group II metabotropic glutamate receptors produces hyper-locomotion in cocaine pre-exposed rats by interactions with dopamine receptors". Neuropharmacology. 55 (4): 555–9. doi:10.1016/j.neuropharm.2008.07.012. PMID 18675831.
- ↑ Sakagami K, Yasuhara A, Chaki S, et al. (April 2008). "Synthesis, in vitro pharmacology, and pharmacokinetic profiles of 2-[1-amino-1-carboxy-2-(9H-xanthen-9-yl)-ethyl]-1-fluorocyclopropanecarboxylic acid and its 6-heptyl ester, a potent mGluR2 antagonist". Bioorg. Med. Chem. 16 (8): 4359–66. doi:10.1016/j.bmc.2008.02.066. PMID 18348906.
This article is issued from Wikipedia - version of the 6/1/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.