Dasolampanel
 | |
| Clinical data | |
|---|---|
| ATC code | None |
| Identifiers | |
| |
| CAS Number | 503294-13-1 |
| PubChem (CID) | 51049972 |
| ChemSpider | 25948207 |
| UNII |
1P85D6BE9K |
| Chemical and physical data | |
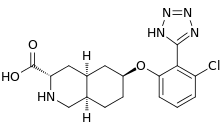
| Formula | C17H20ClN5O3 |
| Molar mass | 377.825 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
Dasolampanel (INN, USAN, code name NGX-426) is an orally bioavailable analog of tezampanel and thereby competitive antagonist of the AMPA and kainate receptors which was under development by Raptor Pharmaceuticals/Torrey Pines Therapeutics for the treatment of chronic pain conditions including neuropathic pain and migraine.[1] It was developed as a follow-on compound to tezampanel, as tezampanel is not bioavailable orally and must be administered by intravenous injection,[2][3] but ultimately neither drug was ever marketed.
See also
References
- ↑ Ian P. Stolerman (31 July 2010). Encyclopedia of Psychopharmacology. Springer Science & Business Media. pp. 514–. ISBN 978-3-540-68698-9.
- ↑ Jes Olesen; Nabih Ramadan (21 August 2008). Innovative Drug Development for Headache Disorders. Oxford University Press. pp. 188–. ISBN 978-0-19-955276-4.
- ↑ Gary S. Firestein; Ralph Budd; Sherine E Gabriel; James R O'Dell; Iain B. McInnes (31 August 2012). Kelley's Textbook of Rheumatology: Expert Consult Premium Edition: Enhanced Online Features. Elsevier Health Sciences. pp. 1031–. ISBN 1-4557-3767-4.
This article is issued from Wikipedia - version of the 4/2/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.