Diazoxide
 | |
| Clinical data | |
|---|---|
| Trade names | Proglycem |
| AHFS/Drugs.com | Monograph |
| Pregnancy category | |
| Routes of administration | Oral, intravenous |
| ATC code | C02DA01 (WHO) V03AH01 (WHO) |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Protein binding | 90% |
| Metabolism | Hepatic oxidation and sulfate conjugation |
| Biological half-life | 21-45 hours |
| Excretion | Renal |
| Identifiers | |
| |
| CAS Number |
364-98-7 |
| PubChem (CID) | 3019 |
| IUPHAR/BPS | 2409 |
| DrugBank |
DB01119 |
| ChemSpider |
2911 |
| UNII |
O5CB12L4FN |
| KEGG |
D00294 |
| ChEBI |
CHEBI:4495 |
| ChEMBL |
CHEMBL181 |
| ECHA InfoCard | 100.006.063 |
| Chemical and physical data | |
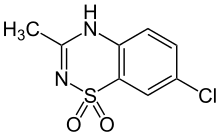
| Formula | C8H7ClN2O2S |
| Molar mass | 230.672 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
| (verify) | |
Diazoxide (INN; brand name Proglycem[1]) is a potassium channel activator, which causes local relaxation in smooth muscle by increasing membrane permeability to potassium ions. This switches off voltage-gated calcium ion channels, preventing calcium flux across the sarcolemma and activation of the contractile apparatus.
In the United States, this agent is typically given in hospital.[2]
Uses
Diazoxide is used as a vasodilator in the treatment of acute hypertension or malignant hypertension.[3]
Diazoxide also inhibits the secretion of insulin from the pancreas, thus it is used to counter hypoglycemia in disease states such as insulinoma (a tumor producing insulin)[4] or congenital hyperinsulinism.
Diazoxide acts as a positive allosteric modulator of the AMPA and kainate receptors, suggesting potential application as a cognitive enhancer.[5]
Side effects
Diazoxide interferes with insulin release through its action on potassium channels.[6] Diazoxide is one of the most potent openers of the K+ ATP channels present on the insulin producing beta cells of the pancreas. Opening these channels leads to hyperpolarization of cell membrane, a decrease in calcium influx, and a subsequently reduced release of insulin.[7] This mechanism of action is the mirror opposite of that of Sulfonylureas, a class of medications used to increase insulin release in Type 2 Diabetics. Therefore, this medicine is not given to non-insulin dependent diabetic patients.
The Food and Drug Administration published a Safety Announcement in July 2015 highlighting the potential for development of pulmonary hypertension in newborns and infants treated with this drug.[2]
See also
- Hydrochlorothiazide (HCTZ)
References
- ↑ Diazoxide, drugs.com
- 1 2 "FDA Drug Safety Communication: FDA warns about a serious lung condition in infants and newborns treated with Proglycem (diazoxide)" (Press release). Food and Drug Administration. July 16, 2015. Retrieved 2015-07-19.
- ↑ van Hamersvelt HW, Kloke HJ, de Jong DJ, Koene RA, Huysmans FT (August 1996). "Oedema formation with the vasodilators nifedipine and diazoxide: direct local effect or sodium retention?". Journal of Hypertension. 14 (8): 1041–5. doi:10.1097/00004872-199608000-00016. PMID 8884561.

- ↑ Huang Q, Bu S, Yu Y, et al. (January 2007). "Diazoxide prevents diabetes through inhibiting pancreatic beta-cells from apoptosis via Bcl-2/Bax rate and p38-beta mitogen-activated protein kinase". Endocrinology. 148 (1): 81–91. doi:10.1210/en.2006-0738. PMID 17053028.

- ↑ Randle, John C.R.; Biton, Catherine; Lepagnol, Jean M. (15 November 1993). "Allosteric potentiation by diazoxide of AMPA receptor currents and synaptic potentials". European Journal of Pharmacology. 247 (3): 257–65. doi:10.1016/0922-4106(93)90193-D. PMID 8307099.

- ↑ Panten, Uwe; Burgfeld, Johanna; Goerke, Frank; Rennicke, Michael; Schwanstecher, Mathias; Wallasch, Andreas; Zünkler, Bernd J.; Lenzen, Sigurd (1989-04-15). "Control of insulin secretion by sulfonylureas, meglitinide and diazoxide in relation to their binding to the sulfonylurea receptor in pancreatic islets". Biochemical Pharmacology. 38 (8): 1217–1229. doi:10.1016/0006-2952(89)90327-4.
- ↑ Doyle, Máire E.; Egan, Josephine M. (2003-03-01). "Pharmacological Agents That Directly Modulate Insulin Secretion". Pharmacological Reviews. 55 (1): 105–131. doi:10.1124/pr.55.1.7. ISSN 1521-0081. PMID 12615955.