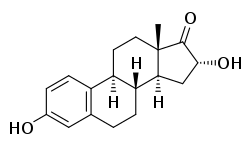
16α-Hydroxyestrone
16α-Hydroxyestrone
 |
| Names |
| IUPAC name
(8R,9S,13S,14S,16R)-3,16-Dihydroxy-13-methyl-7,8,9,11,12,14,15,16-octahydro-6H-cyclopenta[a]phenanthren-17-one |
| Other names
Hydroxyestrone; 16-Hydroxyestrone |
| Identifiers |
| |
566-76-7 |
| 3D model (Jmol) |
Interactive image |
| ChemSpider |
103012 |
| PubChem |
115116 |
InChI=1S/C18H22O3/c1-18-7-6-13-12-5-3-11(19)8-10(12)2-4-14(13)15(18)9-16(20)17(18)21/h3,5,8,13-16,19-20H,2,4,6-7,9H2,1H3/t13-,14-,15+,16-,18+/m1/s1
|
C[C@]12CC[C@H]3[C@H]([C@@H]1C[C@H](C2=O)O)CCC4=C3C=CC(=C4)O
|
| Properties |
| |
C18H22O3 |
| Molar mass |
286.37 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). |
|
| Infobox references |
|
|
16α-Hydroxyestrone (16α-OH-E1), or hydroxyestrone, also known as estra-1,3,5(10)-trien-3,16α-diol-17-one, is an endogenous steroidal estrogen and a major metabolite of estrone, as well as an intermediate in the biosynthesis of estriol.[1][2] It is a potent estrogen similarly to estrone, and it has been suggested that the ratio of 16α-hydroxyestrone to 2-hydroxyestrone, the latter being much less estrogenic in comparison and even antiestrogenic in the presence of more potent estrogens like estradiol, may be involved in the pathophysiology of breast cancer.[1] Conversely, 16α-hydroxyestrone may help to protect against osteoporosis.[1] In contrast to estradiol, the binding of 16α-hydroxyestrone to the estrogen receptor is, uniquely, covalent and irreversible, and genotoxicity and aberrant hyperproliferations may result.[3] A diacetate ester of 16α-hydroxyestrone, hydroxyestrone diacetate, has been marketed and is used medically as an estrogen in Europe.[4][5]
See also
References
|
|---|
|
| ER | Agonists |
- Steroidal: 1-Keto-1,2,3,4-tetrahydrophenanthrene
- 3α-Androstanediol
- 3β-Androstanediol
- 3α-Hydroxytibolone
- 3β-Hydroxytibolone
- 4-Androstenediol
- 4-Androstenedione
- 4-Hydroxyestradiol
- 4-Hydroxyestrone
- 5-Androstenediol
- 7-Oxo-DHEA
- 7α-Hydroxy-DHEA
- 7β-Hydroxyepiandrosterone
- 8,9-Dehydroestrone
- 8β-VE2
- 16α-Hydroxy-DHEA
- 16α-Hydroxyestrone
- 16α-Iodo-E2
- 16α-LE2
- 16β,17α-Epiestriol (16β-hydroxy-17α-estradiol)
- 17α-Dihydroequilenin
- 17α-Dihydroequilin
- 17α-Epiestriol (16α-hydroxy-17α-estradiol)
- 17β-Dihydroequilenin
- 17β-Dihydroequilin
- Abiraterone
- Abiraterone acetate
- 17α-Estradiol (alfatradiol)
- Alestramustine
- Almestrone
- Anabolic steroids (e.g., testosterone and esters, methyltestosterone, metandienone (methandrostenolone), nandrolone and esters, many others; via estrogenic metabolites)
- Atrimustine
- Bolandiol
- Bolandiol dipropionate
- Butolame
- Clomestrone
- Cloxestradiol
- DHEA
- DHEA-S
- Digitoxin (digitalis)
- Diosgenin
- Epiestriol (16β-epiestriol, 16β-hydroxy-17β-estradiol)
- Epimestrol
- Equilenin
- Equilin
- Estetrol
- Estradiol
- Estramustine
- Estramustine phosphate
- Estrapronicate
- Estrazinol
- Estriol
- Estrofurate
- Estromustine
- Estrone
- Etamestrol (eptamestrol)
- Ethinyl estradiol
- Ethinyl estriol
- Etynodiol diacetate
- Guggulsterone
- Hexolame
- Hydroxyestrone diacetate
- Mestranol
- Methylestradiol
- Moxestrol
- Mytatrienediol
- Nilestriol
- Noretynodrel
- Orestrate
- Pentolame
- Phytosterols (e.g., β-sitosterol, campesterol, stigmasterol)
- Polyestradiol phosphate
- Prodiame
- Prolame
- Promestriene
- Quinestradol
- Quinestrol
- Non-steroidal: (R,R)-THC
- (S,S)-THC
- 2,8-DHHHC
- Allenoic acid
- Alternariol
- Anethole
- Anol
- Benzestrol
- Bifluranol
- Biochanin A
- Bisdehydrodoisynolic acid
- Carbestrol
- Chalconoids (e.g., isoliquiritigenin, phloretin, phlorizin (phloridzin), wedelolactone)
- Coumestans (e.g., coumestrol, psoralidin)
- Deoxymiroestrol
- Dianethole
- Dianol
- Diarylpropionitrile
- Dieldrin
- Dienestrol
- Diethylstilbestrol
- Dimestrol (dianisylhexene)
- Dimethylallenolic acid
- Doisynoestrol (fenocycline)
- Doisynolic acid
- Efavirenz
- Endosulfan
- ERB-196 (WAY-202196)
- Estrobin (DBE)
- Fenarimol
- Fenestrel
- FERb 033
- Flavonoids (incl. 7,8-DHF, 8-prenylnaringenin, apigenin, baicalein, baicalin, calycosin, catechin, daidzein, daidzin, ECG, EGCG, epicatechin, equol, formononetin, glabrene, glabridin, genistein, genistin, glycitein, kaempferol, liquiritigenin, mirificin, myricetin, naringenin, pinocembrin, prunetin, puerarin, quercetin, tectoridin, tectorigenin)
- Fosfestrol (diethylstilbestrol diphosphate)
- Furostilbestrol (diethylstilbestrol difuroate)
- GTx-758
- Hexestrol
- ICI-85966 (Stilbostat)
- Lavender oil
- Lignans (e.g., enterodiol, enterolactone)
- Mestilbol
- Metalloestrogens (e.g., cadmium)
- Methallenestril
- Methestrol
- Methestrol dipropionate
- Methiocarb
- Methoxychlor
- Miroestrol
- Nyasol (cis-hinokiresinol)
- Paroxypropione
- Pentafluranol
- Phenestrol
- Photoanethole
- Prinaberel (ERB-041, WAY-202041)
- Propylpyrazoletriol
- Resorcylic acid lactones (e.g., zearalanone, zearalenol, zearalenone, zeranol (α-zearalanol), taleranol (teranol, β-zearalanol))
- Quadrosilan
- SC-4289
- SKF-82,958
- Stilbenoids (e.g., resveratrol)
- Synthetic xenoestrogens (e.g., alkylphenols, bisphenols (e.g., BPA, BPF, BPS), DDT, parabens, PBBs, PHBA, phthalates, PCBs)
- Terfluranol
- WAY-166818
- WAY-200070
- Triphenylchlorethylene
- Triphenylmethylethylene
- WAY-214156
|
|---|
| | |
|---|
| Antagonists | |
|---|
|
|---|
|
| GPER | |
|---|
|
See also: Androgenics • Glucocorticoidics • Mineralocorticoidics • Progestogenics • Steroid hormone metabolism modulators |
