Dihydrotestosterone
 | |
 | |
| Clinical data | |
|---|---|
| Pregnancy category |
|
| Routes of administration | Intramuscular, transdermal |
| ATC code | A14AA01 (WHO) |
| Pharmacokinetic data | |
| Bioavailability | Oral: 0–2% |
| Metabolism | Hepatic |
| Excretion | Renal |
| Identifiers | |
| |
| CAS Number |
521-18-6 |
| PubChem (CID) | 10635 |
| IUPHAR/BPS | 2856 |
| DrugBank |
DB02901 |
| ChemSpider |
10189 |
| UNII |
08J2K08A3Y |
| ChEBI |
CHEBI:16330 |
| ChEMBL |
CHEMBL27769 |
| Chemical and physical data | |
| Formula | C19H30O2 |
| Molar mass | 290.442 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
| | |
Dihydrotestosterone (DHT), or 5α-dihydrotestosterone (5α-DHT), also known as 5α-androstan-17β-ol-3-one, is an endogenous androgen sex steroid and hormone. The enzyme 5α-reductase catalyzes the formation of DHT from testosterone in certain tissues including the prostate gland, seminal vesicles, epididymides, skin, hair follicles, liver, and brain. This enzyme mediates reduction of the C4-5 double bond of testosterone. Relative to testosterone, DHT is considerably more potent as an agonist of the androgen receptor (AR).
Biological activity
DHT has an affinity (Kd) of 0.25 to 0.5 nM for the human AR, which is about 2- to 3-fold higher than that of testosterone (Kd = 0.4 to 1.0 nM)[1] and 15–30 times higher than that of adrenal androgens.[2] The dissociation rate of DHT from the AR is 5-fold slower than that of testosterone.[3] The EC50 of DHT for activation of the AR is 0.13 nM, which is about 5-fold higher than that of testosterone (EC50 = 0.66 nM).[4] In bioassays, DHT has been found to be 2.5- to 10-fold more potent than testosterone.[1]
The terminal half-life of DHT in the body (53 minutes) is longer than that of testosterone (34 minutes), and this may account for some of the difference in their potency.[5] A study of transdermal DHT and testosterone treatment reported terminal half-lives of 2.83 hours and 1.29 hours, respectively.[6]
Biological function
Sexual development
During male embryogenesis DHT has an essential role in the formation of the male external genitalia, while in the adult male DHT acts as the primary androgen in the prostate gland, seminal vesicles, skin, and hair follicles.[7]
An example illustrating the significance of DHT for the development of secondary sex characteristics is congenital 5α-reductase type II deficiency. This genetic mutation can result in pseudohermaphroditism.[8] The condition typically presents with underdeveloped male genitalia and prostate. Males with this condition are often raised as girls due to their lack of conspicuous male genitalia.[8] At the onset of puberty, although their DHT levels remain very low, their testosterone levels elevate normally. Their musculature develops like that of other male adults. After puberty, men with this condition have a large deficiency of pubic and body hair and reportedly no incidence of androgenic alopecia (pattern hair loss).[9] They also reportedly have no incidence of prostate cancer.[10]
Unlike other androgens such as testosterone, DHT cannot be converted by the enzyme aromatase into an estrogen like estradiol. Therefore, it is frequently used in research settings to distinguish between the effects of testosterone caused by binding to the AR and those caused by testosterone's conversion to estradiol and subsequent binding to and activation of estrogen receptors.[11]
Pathology
DHT produced locally at the site of hair follicles by 5α-reductase, and not systemic DHT, is the primary causal factor in male androgenic alopecia, although the pathology regarding this phenomenon is poorly understood.[12][13] In the case of female androgenic alopecia, on the other hand, the situation is more complex, and DHT is only one of several possible causes.[14] Women with increased levels of DHT may develop symptoms of hyperandrogenism such as certain androgynous masculine secondary sex characteristics, including a deepened voice and facial hair. In men, prostate growth and differentiation are highly dependent on androgens, especially DHT, and DHT is involved in the pathogenesis of benign prostatic hyperplasia (BPH) and prostate cancer.[15]
Management
5α-Reductase inhibitors like finasteride and dutasteride, which inactivate the 5α-reductase enzyme and block the formation of DHT, are commonly used for the treatment of two DHT-related conditions, androgenic alopecia and BPH. Both finasteride and dutasteride are approved for the treatment of BPH and androgenic alopecia. Dutasteride is three times more potent than finasteride in inhibiting the type II enzyme and 100 times more potent than finasteride in inhibiting the type I form of the DHT-producing enzyme. Both finasteride and dutasteride are potent inhibitors of the third isotype of the enzyme.[16]
Acne, hirsutism (excessive hair growth), and seborrhea are also DHT-related conditions, and 5α-reductase inhibitors may be used to treat these conditions as well.[17] In addition, antiandrogens like cyproterone acetate, spironolactone, and bicalutamide, as well as estrogens like ethinyl estradiol (which are functional antiandrogens), may also be used to treat these conditions.[17][18]
Biochemistry
Biosynthesis
DHT is synthesized from testosterone by the enzyme 5α-reductase.[19] In males, approximately 5% of testosterone undergoes 5α-reduction into DHT.
Metabolism
DHT is inactivated in the liver and extrahepatic tissues like the skin into 3α-androstanediol and 3β-androstanediol by the enzymes 3α-hydroxysteroid dehydrogenase and 3β-hydroxysteroid dehydrogenase, respectively.[20] These metabolites are in turn converted, respectively, into androsterone and epiandrosterone, then conjugated (via glucuronidation and/or sulfation), released into circulation, and excreted in urine.
Unlike testosterone, DHT cannot be aromatized into an estrogen, and for this reason, has no propensity for estrogenic effects.[21]
Levels
Serum DHT levels are about 10% of those of testosterone, but levels in the prostate gland are 5- to 10-fold higher than those of testosterone due to a more than 90% conversion of testosterone into DHT by locally expressed 5α-reductase.[22] For this reason, and in addition to the fact that DHT is much more potent as an AR agonist than is testosterone,[1] DHT is considered to be the major androgen of the prostate gland.[22]
Medical use
DHT is available in pharmaceutical formulations for medical use as an androgen or anabolic-androgenic steroid (AAS).[23] When used as a drug, it is referred to as androstanolone (INN) or as stanolone (BAN).[23][24][25] The availability of pharmaceutical DHT is limited; it is not available in the United States or Canada,[26][27] but is available in certain European countries, including the United Kingdom, Germany, France, Spain, Italy, Belgium, and Luxembourg.[25][28] Brand names of DHT include Anaboleen, Anabolex, Anaprotin (UK), Andractim (formerly AndroGel-DHT) (FR, BE, LU), Androlone, Apeton, Gelovit (ES), Neodrol, Ophtovital, (DE), Pesomax (IT), Stanaprol, and Stanolone, among others.[23][24][25][28][29] The available formulations of DHT include buccal or sublingual tablets (Anabolex, Stanolone), topical gels (Andractim, Gelovit, Ophtovital), and, as esters in oil, injectables like dihydrotestosterone propionate (Pesomax) and dihydrotestosterone valerate (Apeton).[23][28][29] Esters of DHT act as prodrugs of DHT in the body and have a long-lasting depot when given via intramuscular injection.[23] Dihydrotestosterone benzoate (Ermalone-Amp, Hermalone, Sarcosan) and dihydrotestosterone enanthate (Anaboleen Depot) are additional DHT esters that are also available for medical use, while a few others, including dihydrotestosterone acetate, dihydrotestosterone butyrate, and dihydrotestosterone formate, were developed but never marketed.[24]
Unlike testosterone and various synthetic AAS, DHT cannot be aromatized, and for this reason, poses no risk of estrogenic side effects like gynecomastia at any dosage.[30] In addition, DHT cannot be metabolized by 5α-reductase (as it is already 5α-reduced), and for this reason, is not potentiated in so-called androgenic tissues like the skin, hair follicles, and prostate gland. This provides exogenous DHT with a greater ratio of anabolic to androgenic effects compared to testosterone, and DHT may be less prone to producing certain skin and hair-related side effects like acne, oily skin, seborrhea, hirsutism (excess facial/body hair growth), and androgenic alopecia (pattern hair loss), as well as prostate enlargement (which can lead to benign prostatic hyperplasia) and an increased risk of prostate cancer.
Pharmaceutical DHT is used mainly in the treatment of male hypogonadism.[28] It was under development in a topical formulation for the treatment of cachexia in cancer patients, and reached phase III clinical trials for this indication, but ultimately was not introduced for this purpose.[28] Although DHT itself has not been approved for the treatment of cachexia, an orally active synthetic derivative of DHT, oxandrolone (2-oxa-17α-methyl-DHT), is approved and used for this indication.[31][32]
Chemistry
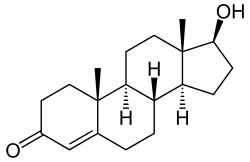
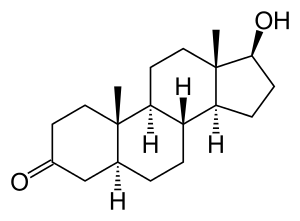
DHT is a 5α-androstane (C19) steroid with a ketone group at the C3 position and a hydroxyl group at the C17β position. It is the derivative of testosterone in which the double bond between the C4 and C5 positions has been reduced or hydrogenated.
Derivatives
Several C17β ester prodrugs of DHT, including androstanolone benzoate, androstanolone enanthate, androstanolone propionate, and androstanolone valerate, have been developed and introduced for medical use as AAS.[24][33]
Synthetic derivatives of DHT used as AAS include mesterolone (1α-methyl-DHT), drostanolone (2α-methyl-DHT), metenolone (1β-methyl-δ1-DHT), stenbolone (2-methyl-δ1-DHT), epitiostanol (2α,3α-epithio-3-deketo-DHT), mepitiostane (a 17-ether prodrug of epitiostanol), 1-testosterone (dihydroboldenone; Δ1-DHT), mesabolone (a 17-ether prodrug of Δ1-DHT), prostanozol (a 17-ether prodrug of the non-17α-methylated analogue of stanozolol), and bolazine (an azine dimer prodrug of a drostanolone-like AAS), as well as the 17α-alkylated derivatives mestanolone (17α-methyl-DHT), methasterone (2α,17α-dimethyl-DHT), oxandrolone (2-oxa-17α-methyl-DHT), oxymetholone (2-hydroxymethylene-17α-methyl-DHT), stanozolol (a 2,3-pyrazole A ring-fused derivative of 17α-methyl-DHT), furazabol (a 2,3-furan A ring-fused derivative of 17α-methyl-DHT), androisoxazole (a 2,3-isoxazole A ring-fused derivative of 17α-methyl-DHT), methylstenbolone (2,17α-dimethyl-δ1-DHT), methyl-1-testosterone (methyldihydroboldenone; 17α-methyl-δ1-DHT), methylepitiostanol (2α,3α-epithio-3-deketo-17α-methyl-DHT), desoxymethyltestosterone (3-deketo-17α-methyl-δ2-DHT), and mebolazine (an azine dimer prodrug of a methasterone-like AAS).[34]
References
- 1 2 3 Ashraf Mozayani; Lionel Raymon (18 September 2011). Handbook of Drug Interactions: A Clinical and Forensic Guide. Springer Science & Business Media. pp. 656–. ISBN 978-1-61779-222-9.
- ↑ Hemat RAS (2004). Principles Of Orthomolecularism. Urotext. p. 426. ISBN 1-903737-05-2.
- ↑ Grino, P. B.; Griffin, J. E.; Wilson, J. D. (1990). "Testosterone at high concentrations interacts with the human androgen receptor similarly to dihydrotestosterone". Endocrinology. 126 (2): 1165–1172. doi:10.1210/endo-126-2-1165. PMID 2298157.
- ↑ Treatise on Water Science, Four-Volume Set. Newnes. 1 September 2010. pp. 1805–. ISBN 978-0-444-53199-5.
- ↑ Bentham Science Publishers (September 1999). Current Pharmaceutical Design. Bentham Science Publishers. pp. 708–.
- ↑ Ashraf Mozayani; Lionel Raymon (15 October 2003). Handbook of Drug Interactions: A Clinical and Forensic Guide. Springer Science & Business Media. pp. 510–. ISBN 978-1-59259-654-6.
- ↑ Amory JK, Anawalt BD, Matsumoto AM, Page ST, Bremner WJ, Wang C, Swerdloff RS, Clark RV (June 2008). "The effect of 5alpha-reductase inhibition with dutasteride and finasteride on bone mineral density, serum lipoproteins, hemoglobin, prostate specific antigen and sexual function in healthy young men". J. Urol. 179 (6): 2333–8. doi:10.1016/j.juro.2008.01.145. PMC 2684818
 . PMID 18423697.
. PMID 18423697. - 1 2 Imperato-McGinley J, Peterson RE, Gautier T, Sturla E (May 1979). "Androgens and the Evolution of Male-Gender Identity among Male Pseudohermaphrodites with 5α-Reductase Deficiency". New England Journal of Medicine. 300: 1233–1237. doi:10.1056/NEJM197905313002201. PMID 431680.
- ↑ Marks LS (2004). "5α-reductase: history and clinical importance". Rev Urol. 6 Suppl 9: S11–21. PMC 1472916
 . PMID 16985920.
. PMID 16985920. - ↑ N. K. Jain; Maqsood Siddiqi; J. H. Weisburger (2006). Protective Effects of Tea on Human Health. CABI. pp. 95–. ISBN 978-1-84593-113-1.
- ↑ Swerdloff RS, Wang C (October 1998). "Dihydrotestosterone: a rationale for its use as a non-aromatizable androgen replacement therapeutic agent". Baillieres Clin. Endocrinol. Metab. 12 (3): 501–6. doi:10.1016/s0950-351x(98)80267-x. PMID 10332569.
- ↑ Nordqvist C (2012-02-23). "What Is DHT (Dihydrotestosterone)? What Is DHT's Role In Baldness?". Medical News Today.
- ↑ "Male Pattern Baldness Causes". Hair Loss Health Center. WebMD, LLC.
- ↑ McAndrews PJ. "Women's Hair Loss / Causes of Hair Loss". American Hair Loss Association.
- ↑ Freedland SJ, Isaacs WB, Platz EA, Terris MK, Aronson WJ, Amling CL, Presti JC, Kane CJ (October 2005). "Prostate size and risk of high-grade, advanced prostate cancer and biochemical progression after radical prostatectomy: a search database study". J. Clin. Oncol. 23 (30): 7546–54. doi:10.1200/JCO.2005.05.025. PMID 16234520.
- ↑ Olsen EA, Hordinsky M, Whiting D, Stough D, Hobbs S, Ellis ML, Wilson T, Rittmaster RS (December 2006). "The importance of dual 5alpha-reductase inhibition in the treatment of male pattern hair loss: results of a randomized placebo-controlled study of dutasteride versus finasteride". J. Am. Acad. Dermatol. 55 (6): 1014–23. doi:10.1016/j.jaad.2006.05.007. PMID 17110217. Lay summary – IAHRS Hair Transplant & Hair Loss Info Center.
- 1 2 Andreas Katsambas; Torello Lotti; Clio Dessinioti; Angelo Massimiliano D'Erme (28 April 2015). European Handbook of Dermatological Treatments. Springer. pp. 1451–1464. ISBN 978-3-662-45139-7.
- ↑ NADIR R. FARID; Evanthia Diamanti-Kandarakis (27 February 2009). Diagnosis and Management of Polycystic Ovary Syndrome. Springer Science & Business Media. pp. 233–242. ISBN 978-0-387-09718-3.
- ↑ Ulrike Blume-Peytavi; David A. Whiting; Ralph M. Trüeb (26 June 2008). Hair Growth and Disorders. Springer Science & Business Media. pp. 161–. ISBN 978-3-540-46911-7.
- ↑ Rizner TL, Lin HK, Peehl DM, Steckelbroeck S, Bauman DR, Penning TM (July 2003). "Human type 3 3alpha-hydroxysteroid dehydrogenase (aldo-keto reductase 1C2) and androgen metabolism in prostate cells". Endocrinology. 144 (7): 2922–32. doi:10.1210/en.2002-0032. PMID 12810547.
- ↑ Irving B. Weiner; Michela Gallagher (2003). Handbook of Psychology, Biological Psychology. John Wiley & Sons. pp. 333–. ISBN 978-0-471-38403-8.
- 1 2 Ian D. Hay; John A. H. Wass (26 January 2009). Clinical Endocrine Oncology. John Wiley & Sons. pp. 37–. ISBN 978-1-4443-0023-9.
- 1 2 3 4 5 Thomas E. Hyde; Marianne S. Gengenbach (2007). Conservative Management of Sports Injuries. Jones & Bartlett Learning. pp. 1100–. ISBN 978-0-7637-3252-3.
- 1 2 3 4 J. Elks (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 640–. ISBN 978-1-4757-2085-3.
- 1 2 3 Index Nominum 2000: International Drug Directory. Taylor & Francis. January 2000. pp. 63–. ISBN 978-3-88763-075-1.
- ↑ "Drugs@FDA: FDA Approved Drug Products". United States Food and Drug Administration. Retrieved 16 November 2016.
- ↑ "Drug Product Database - Health Canada". Health Canada. Retrieved 13 November 2016.
- 1 2 3 4 5 http://webcache.googleusercontent.com/search?q=cache:gUrsCW-RPpgJ:adisinsight.springer.com/drugs/800011409+&cd=1&hl=en&ct=clnk&gl=us
- 1 2 Paul Heinz List; Ludwig Hörhammer (12 March 2013). Chemikalien und Drogen: Teil B: R, S. Springer-Verlag. pp. 523–. ISBN 978-3-642-66377-2.
- ↑ Paul V. Malven (12 January 1993). Mammalian Neuroendocrinology. CRC Press. pp. 228–. ISBN 978-0-8493-8757-9.
- ↑ Marcia Nelms; Kathryn P. Sucher; Karen Lacey; Sara Long Roth (16 June 2010). Nutrition Therapy and Pathophysiology. Cengage Learning. pp. 766–. ISBN 1-133-00809-7.
- ↑ Giovanni Mantovani (6 October 2007). Cachexia and Wasting: A Modern Approach. Springer Science & Business Media. pp. 673–. ISBN 978-88-470-0552-5.
- ↑ I.K. Morton; Judith M. Hall (6 December 2012). Concise Dictionary of Pharmacological Agents: Properties and Synonyms. Springer Science & Business Media. pp. 261–. ISBN 978-94-011-4439-1.
- ↑ William Llewellyn (2011). Anabolics. Molecular Nutrition Llc. pp. 23–25. ISBN 978-0-9828280-1-4.