GABAB receptor
| gamma-aminobutyric acid (GABA) B receptor, 1 | |
|---|---|
| Identifiers | |
| Symbol | GABBR1 |
| Entrez | 2550 |
| HUGO | 4070 |
| OMIM | 603540 |
| RefSeq | NM_021905 |
| UniProt | Q9UBS5 |
| Other data | |
| Locus | Chr. 6 p21.3 |
| gamma-aminobutyric acid (GABA) B receptor, 2 | |
|---|---|
| Identifiers | |
| Symbol | GABBR2 |
| Alt. symbols | GPR51 |
| Entrez | 9568 |
| HUGO | 4507 |
| OMIM | 607340 |
| RefSeq | NM_005458 |
| UniProt | O75899 |
| Other data | |
| Locus | Chr. 9 q22.1-22.3 |
GABAB receptors (GABABR) are metabotropic transmembrane receptors for gamma-aminobutyric acid (GABA) that are linked via G-proteins to potassium channels.[1] The changing potassium concentrations hyperpolarize the cell at the end of an action potential. The reversal potential of the GABAB-mediated IPSP is -100 mV, which is much more hyperpolarized than the GABAA IPSP. GABAB receptors are found in the central as well as in the autonomic division of the peripheral nervous system.
The receptors were first named in 1981 when their distribution in the CNS was determined, which was determined by Norman Bowery and his team using radioactively labelled baclofen.[2]
Functions
They can stimulate the opening of K+ channels which brings the neuron closer to the equilibrium potential of K+, hyperpolarising the neuron. This prevents voltage-gated sodium channels from opening, action potentials from firing, and VDCCs from opening, and so stops neurotransmitter release. Thus GABAB receptors are considered inhibitory receptors.
GABAB receptors can also reduce the activity of adenylyl cyclase and decrease the cell’s conductance to Ca2+.
GABAB receptors are involved in behavioral actions of ethanol,[3] gamma-Hydroxybutyric acid (GHB),[4] and possibly in pain.[5] Recent research suggests that these receptors may play an important developmental role.[6]
Structure
GABAB Receptors are similar in structure to and in the same receptor family with metabotropic glutamate receptors.[7] There are two subtypes of the receptor, GABAB1 and GABAB2,[8] and these appear to assemble as heterodimers in neuronal membranes by linking up by their intracellular C termini.[7]
It is speculated that binding of GABA causes the subunits to swing shut around the agonist like a venus fly trap.
Ligands
Agonists
- GABA
- Baclofen is a GABA analogue which acts as a selective agonist of GABAB receptors, and is used as a muscle relaxant. However, it can aggravate absence seizures, and so is not used in epilepsy.
- gamma-Hydroxybutyrate (GHB)
- Phenibut
- Isovaline
- 3-Aminopropylphosphinic acid
- Lesogaberan
- SKF-97541: 3-Aminopropyl(methyl)phosphinic acid, 10x more potent than baclofen as GABAB agonist, but also GABAC antagonist
- CGP-44532
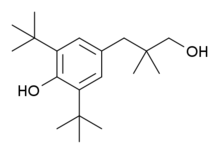
Positive Allosteric Modulators
Antagonists
- Homotaurine [13]
- Ginsenosides [14]
- 2-OH-saclofen
- Fasoracetam
- Saclofen
- Phaclofen
- SCH-50911
- 2-Phenethylamine
- CGP-35348
- CGP-52432: 3-([(3,4-Dichlorophenyl)methyl]amino]propyl) diethoxymethyl)phosphinic acid, CAS# 139667-74-6
- CGP-55845: (2S)-3-([(1S)-1-(3,4-Dichlorophenyl)ethyl]amino-2-hydroxypropyl)(phenylmethyl)phosphinic acid, CAS# 149184-22-5
- SGS-742 [15][16]
See also
References
- ↑ Chen K, Li HZ, Ye N, Zhang J, Wang JJ (2005). "Role of GABAB receptors in GABA and baclofen-induced inhibition of adult rat cerebellar interpositus nucleus neurons in vitro". Brain Res Bull. 67 (4): 310–8. doi:10.1016/j.brainresbull.2005.07.004. PMID 16182939.
- ↑ Hill, DR; Bowery, NG (12 March 1981). "3H-baclofen and 3H-GABA bind to bicuculline-insensitive GABA B sites in rat brain.". Nature. 290 (5802): 149–52. PMID 6259535.
- ↑ Dzitoyeva S, Dimitrijevic N, Manev H (2003). "Gamma-aminobutyric acid B receptor 1 mediates behavior-impairing actions of alcohol in Drosophila: adult RNA interference and pharmacological evidence". Proc Natl Acad Sci USA. 100 (9): 5485–90. Bibcode:2003PNAS..100.5485D. doi:10.1073/pnas.0830111100. PMC 154371
 . PMID 12692303.
. PMID 12692303. - ↑ Dimitrijevic N, Dzitoyeva S, Satta R, Imbesi M, Yildiz S, Manev H (2005). "Drosophila GABA(B) receptors are involved in behavioral effects of gamma-hydroxybutyric acid (GHB)". Eur J Pharmacol. 519 (3): 246–52. doi:10.1016/j.ejphar.2005.07.016. PMID 16129424.
- ↑ Manev H, Dimitrijevic N (2004). "Drosophila model for in vivo pharmacological analgesia research". Eur J Pharmacol. 491 (2–3): 207–8. doi:10.1016/j.ejphar.2004.03.030. PMID 15140638.
- ↑ Dzitoyeva S, Gutnov A, Imbesi M, Dimitrijevic N, Manev H (2005). "Developmental role of GABAB(1) receptors in Drosophila". Brain Res Dev Brain Res. 158 (1–2): 111–4. doi:10.1016/j.devbrainres.2005.06.005. PMID 16054235.
- 1 2 MRC (Medical Research Council). 2003. Glutamate receptors: Structures and functions. University of Brisotol Centre for Synaptic Plasticity.
- ↑ Purves D., Augustine G.J., Fitzpatrick D., Katz L.C., LaMantia A.S., McNamara J.O., and Williams S.M. 2001. Neuroscience, Second Edition. Sinauer Associates, Inc.
- ↑ Urwyler S, Mosbacher J, Lingenhoehl K, Heid J, Hofstetter K, Froestl W, Bettler B, Kaupmann K (November 2001). "Positive allosteric modulation of native and recombinant gamma-aminobutyric acid(B) receptors by 2,6-Di-tert-butyl-4-(3-hydroxy-2,2-dimethyl-propyl)-phenol (CGP7930) and its aldehyde analog CGP13501". Mol. Pharmacol. 60 (5): 963–71. PMID 11641424.
- ↑ Adams CL, Lawrence AJ (2007). "CGP7930: a positive allosteric modulator of the GABAB receptor". CNS Drug Rev. 13 (3): 308–16. doi:10.1111/j.1527-3458.2007.00021.x. PMID 17894647.
- ↑ Paterson NE, Vlachou S, Guery S, Kaupmann K, Froestl W, Markou A (July 2008). "Positive modulation of GABA(B) receptors decreased nicotine self-administration and counteracted nicotine-induced enhancement of brain reward function in rats". J. Pharmacol. Exp. Ther. 326 (1): 306–14. doi:10.1124/jpet.108.139204. PMC 2574924
 . PMID 18445779.
. PMID 18445779. - ↑ Urwyler S, Pozza MF, Lingenhoehl K, Mosbacher J, Lampert C, Froestl W, Koller M, Kaupmann K (October 2003). "N,N'-Dicyclopentyl-2-methylsulfanyl-5-nitro-pyrimidine-4,6-diamine (GS39783) and structurally related compounds: novel allosteric enhancers of gamma-aminobutyric acidB receptor function". J. Pharmacol. Exp. Ther. 307 (1): 322–30. doi:10.1124/jpet.103.053074. PMID 12954816.
- ↑ Giotti A, Luzzi S, Spagnesi S, Zilletti L (1983). "Homotaurine: a GABAB antagonist in guinea-pig ileum.". Br. J. Pharmacol. 79: 855–62. doi:10.1111/j.1476-5381.1983.tb10529.x. PMC 2044932
 . PMID 6652358.
. PMID 6652358. - ↑ Kimura T, Saunders PA, Kim HS, Rheu HM, Oh KW, Ho IK (1994). "Interactions of ginsenosides with ligand-bindings of GABA(A) and GABA(B) receptors". General Pharmacology. 25 (1): 193–9. doi:10.1016/0306-3623(94)90032-9. PMID 8026706.
- ↑ Froestl W, Gallagher M, Jenkins H, Madrid A, Melcher T, Teichman S, Mondadori CG, Pearlman R (October 2004). "SGS742: the first GABA(B) receptor antagonist in clinical trials". Biochemical Pharmacology. 68 (8): 1479–87. doi:10.1016/j.bcp.2004.07.030. PMID 15451390.
- ↑ Bullock R (January 2005). "SGS-742 Novartis". Current Opinion in Investigational Drugs (London, England : 2000). 6 (1): 108–13. PMID 15675610.