Silicon carbide
 | |
| Names | |
|---|---|
| Preferred IUPAC name
Silicon carbide | |
| Other names
Carborundum Moissanite | |
| Identifiers | |
| 409-21-2 | |
| 3D model (Jmol) | Interactive image |
| ChEBI | CHEBI:29390 |
| ChemSpider | 9479 |
| ECHA InfoCard | 100.006.357 |
| EC Number | 206-991-8 |
| 13642 | |
| MeSH | Silicon+carbide |
| PubChem | 9863 |
| RTECS number | VW0450000 |
| |
| |
| Properties | |
| CSi | |
| Molar mass | 40.10 g·mol−1 |
| Appearance | Yellow to green to bluish-black, iridescent crystals[1] |
| Density | 3.21 g·cm−3 (all polytypes)[2] |
| Melting point | 2,730 °C (4,950 °F; 3,000 K) (decomposes) |
| Electron mobility | ~900 cm2/V·s (all polytypes) |
| Refractive index (nD) |
2.55 (infrared; all polytypes)[3] |
| Hazards | |
| EU classification (DSD) |
Not listed |
| NFPA 704 | |
| US health exposure limits (NIOSH): | |
| PEL (Permissible) |
TWA 15 mg/m3 (total) TWA 5 mg/m3 (resp)[1] |
| REL (Recommended) |
TWA 10 mg/m3 (total) TWA 5 mg/m3 (resp)[1] |
| IDLH (Immediate danger) |
N.D.[1] |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Silicon carbide (SiC), also known as carborundum /kɑːrbəˈrʌndəm/, is a compound of silicon and carbon with chemical formula SiC. It occurs in nature as the extremely rare mineral moissanite. Silicon carbide powder has been mass-produced since 1893 for use as an abrasive. Grains of silicon carbide can be bonded together by sintering to form very hard ceramics that are widely used in applications requiring high endurance, such as car brakes, car clutches and ceramic plates in bulletproof vests. Electronic applications of silicon carbide such as light-emitting diodes (LEDs) and detectors in early radios were first demonstrated around 1907. SiC is used in semiconductor electronics devices that operate at high temperatures or high voltages, or both. Large single crystals of silicon carbide can be grown by the Lely method; they can be cut into gems known as synthetic moissanite. Silicon carbide with high surface area can be produced from SiO2 contained in plant material.
Discovery and early production
Early experiments
Non-systematic, less-recognized, and often unverified syntheses of silicon carbide include
- J. J. Berzelius's reduction of potassium fluorosilicate by potassium (1810)
- César-Mansuète Despretz's passing an electric current through a carbon rod embedded in sand (1849)
- Robert Sydney Marsden's dissolution of silica in molten silver in a graphite crucible (1881)
- Paul Schuetzenberger's heating of a mixture of silicon and silica in a graphite crucible (1881)
- Albert Colson's heating of silicon under a stream of ethylene (1882).[4]
Wide-scale production

Wide-scale production is credited to Edward Goodrich Acheson in 1890.[5] Acheson was attempting to prepare artificial diamonds when he heated a mixture of clay (aluminum silicate) and powdered coke (carbon) in an iron bowl. He called the blue crystals that formed carborundum, believing it to be a new compound of carbon and aluminum, similar to corundum. In 1893, Henri Moissan discovered the very rare naturally-occurring SiC mineral while examining rock samples found in the Canyon Diablo meteorite in Arizona. The mineral was named moissanite in his honor. Moissan also synthesized SiC by several routes, including dissolution of carbon in molten silicon, melting a mixture of calcium carbide and silica, and by reducing silica with carbon in an electric furnace.
Acheson patented the method for making silicon carbide powder on February 28, 1893.[6] Acheson also developed the electric batch furnace by which SiC is still made today and formed the Carborundum Company to manufacture bulk SiC, initially for use as an abrasive.[7] In 1900 the company settled with the Electric Smelting and Aluminum Company when a judge's decision gave "priority broadly" to its founders "for reducing ores and other substances by the incandescent method".[8] It is said that Acheson was trying to dissolve carbon in molten corundum (alumina) and discovered the presence of hard, blue-black crystals which he believed to be a compound of carbon and corundum: hence carborundum. It may be that he named the material "carborundum" by analogy to corundum, which is another very hard substance (9 on the Mohs scale).
The first use of SiC was as an abrasive. This was followed by electronic applications. In the beginning of the 20th century, silicon carbide was used as a detector in the first radios.[9] In 1907 Henry Joseph Round produced the first LED by applying a voltage to a SiC crystal and observing yellow, green and orange emission at the cathode. Those experiments were later repeated by O. V. Losev in the Soviet Union in 1923.[10]
Natural occurrence

Naturally occurring moissanite is found in only minute quantities in certain types of meteorite and in corundum deposits and kimberlite. Virtually all the silicon carbide sold in the world, including moissanite jewels, is synthetic. Natural moissanite was first found in 1893 as a small component of the Canyon Diablo meteorite in Arizona by Dr. Ferdinand Henri Moissan, after whom the material was named in 1905.[11] Moissan's discovery of naturally occurring SiC was initially disputed because his sample may have been contaminated by silicon carbide saw blades that were already on the market at that time.[12]
While rare on Earth, silicon carbide is remarkably common in space. It is a common form of stardust found around carbon-rich stars, and examples of this stardust have been found in pristine condition in primitive (unaltered) meteorites. The silicon carbide found in space and in meteorites is almost exclusively the beta-polymorph. Analysis of SiC grains found in the Murchison meteorite, a carbonaceous chondrite meteorite, has revealed anomalous isotopic ratios of carbon and silicon, indicating an origin from outside the solar system; 99% of these SiC grains originate around carbon-rich asymptotic giant branch stars.[13] SiC is commonly found around these stars as deduced from their infrared spectra.[14]
Production
Because of the rarity of natural moissanite, most silicon carbide is synthetic. It is used as an abrasive, and more recently as a semiconductor and diamond simulant of gem quality. The simplest manufacturing process is to combine silica sand and carbon in an Acheson graphite electric resistance furnace at a high temperature, between 1,600 °C (2,910 °F) and 2,500 °C (4,530 °F). Fine SiO2 particles in plant material (e.g. rice husks) can be converted to SiC by heating in the excess carbon from the organic material.[15] The silica fume, which is a byproduct of producing silicon metal and ferrosilicon alloys, also can be converted to SiC by heating with graphite at 1,500 °C (2,730 °F).[16]

The material formed in the Acheson furnace varies in purity, according to its distance from the graphite resistor heat source. Colorless, pale yellow and green crystals have the highest purity and are found closest to the resistor. The color changes to blue and black at greater distance from the resistor, and these darker crystals are less pure. Nitrogen and aluminium are common impurities, and they affect the electrical conductivity of SiC.[17]
Pure silicon carbide can be made by the so-called Lely process,[18] in which SiC powder is sublimated into high-temperature species of silicon, carbon, silicon dicarbide (SiC2), and disilicon carbide (Si2C) in an argon gas ambient at 2500 °C and redeposited into flake-like single crystals,[19] sized up to 2×2 cm, at a slightly colder substrate. This process yields high-quality single crystals, mostly of 6H-SiC phase (because of high growth temperature). A modified Lely process involving induction heating in graphite crucibles yields even larger single crystals of 4 inches (10 cm) in diameter, having a section 81 times larger compared to the conventional Lely process.[20] Cubic SiC is usually grown by the more expensive process of chemical vapor deposition (CVD).[17][21] Homoepitaxial and heteroepitaxial SiC layers can be grown employing both gas and liquid phase approaches.[22] Pure silicon carbide can also be prepared by the thermal decomposition of a polymer, poly(methylsilyne), under an inert atmosphere at low temperatures. Relative to the CVD process, the pyrolysis method is advantageous because the polymer can be formed into various shapes prior to thermalization into the ceramic.[23][24][25][26]
Structure and properties
 |
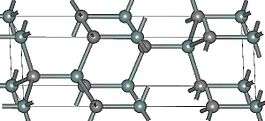 |
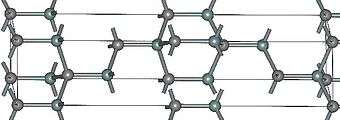 |
| (β)3C-SiC | 4H-SiC | (α)6H-SiC |
Silicon carbide exists in about 250 crystalline forms.[27] The polymorphism of SiC is characterized by a large family of similar crystalline structures called polytypes. They are variations of the same chemical compound that are identical in two dimensions and differ in the third. Thus, they can be viewed as layers stacked in a certain sequence.[28]
Alpha silicon carbide (α-SiC) is the most commonly encountered polymorph; it is formed at temperatures greater than 1700 °C and has a hexagonal crystal structure (similar to Wurtzite). The beta modification (β-SiC), with a zinc blende crystal structure (similar to diamond), is formed at temperatures below 1700 °C.[29] Until recently, the beta form has had relatively few commercial uses, although there is now increasing interest in its use as a support for heterogeneous catalysts, owing to its higher surface area compared to the alpha form.
| Polytype | 3C (β) | 4H | 6H (α) |
|---|---|---|---|
| Crystal structure | Zinc blende (cubic) | Hexagonal | Hexagonal |
| Space group | T2d-F43m | C46v-P63mc | C46v-P63mc |
| Pearson symbol | cF8 | hP8 | hP12 |
| Lattice constants (Å) | 4.3596 | 3.0730; 10.053 | 3.0810; 15.12 |
| Density (g/cm3) | 3.21 | 3.21 | 3.21 |
| Bandgap (eV) | 2.36 | 3.23 | 3.05 |
| Bulk modulus (GPa) | 250 | 220 | 220 |
| Thermal conductivity (W m−1K−1)
@ 300K (see [30] for temp. dependence) |
360 | 370 | 490 |
Pure SiC is colorless. The brown to black color of the industrial product results from iron impurities. The rainbow-like luster of the crystals is caused by a passivation layer of silicon dioxide that forms on the surface.
The high sublimation temperature of SiC (approximately 2700 °C) makes it useful for bearings and furnace parts. Silicon carbide does not melt at any known temperature. It is also highly inert chemically. There is currently much interest in its use as a semiconductor material in electronics, where its high thermal conductivity, high electric field breakdown strength and high maximum current density make it more promising than silicon for high-powered devices.[31] SiC also has a very low coefficient of thermal expansion (4.0 × 10−6/K) and experiences no phase transitions that would cause discontinuities in thermal expansion.[17]
Electrical conductivity
Silicon carbide is a semiconductor, which can be doped n-type by nitrogen or phosphorus and p-type by beryllium, boron, aluminium, or gallium.[3] Metallic conductivity has been achieved by heavy doping with boron, aluminium or nitrogen.
Superconductivity has been detected in 3C-SiC:Al, 3C-SiC:B and 6H-SiC:B at the same temperature of 1.5 K.[29][32] A crucial difference is however observed for the magnetic field behavior between aluminium and boron doping: SiC:Al is type-II, same as Si:B. On the contrary, SiC:B is type-I. In attempt to explain this difference, it was noted that Si sites are more important than carbon sites for superconductivity in SiC. Whereas boron substitutes carbon in SiC, Al substitutes Si sites. Therefore, Al and B "see" different environments that might explain different properties of SiC:Al and SiC:B.[33]
Uses
_disk.jpg)
.jpg)

Abrasive and cutting tools
In the arts, silicon carbide is a popular abrasive in modern lapidary due to the durability and low cost of the material. In manufacturing, it is used for its hardness in abrasive machining processes such as grinding, honing, water-jet cutting and sandblasting. Particles of silicon carbide are laminated to paper to create sandpapers and the grip tape on skateboards.[34]
In 1982 an exceptionally strong composite of aluminium oxide and silicon carbide whiskers was discovered. Development of this laboratory-produced composite to a commercial product took only three years. In 1985, the first commercial cutting tools made from this alumina and silicon carbide whisker-reinforced composite were introduced to the market.[35]
Structural material
In the 1980s and 1990s, silicon carbide was studied in several research programs for high-temperature gas turbines in Europe, Japan and the United States. The components were intended to replace nickel superalloy turbine blades or nozzle vanes. However, none of these projects resulted in a production quantity, mainly because of its low impact resistance and its low fracture toughness.[36]
Like other hard ceramics (namely alumina and boron carbide), silicon carbide is used in composite armor (e.g. Chobham armor), and in ceramic plates in bulletproof vests. Dragon Skin, which was produced by Pinnacle Armor, used disks of silicon carbide.[37]
Silicon carbide is used as a support and shelving material in high temperature kilns such as for firing ceramics, glass fusing, or glass casting. SiC kiln shelves are considerably lighter and more durable than traditional alumina shelves.[38]
In December 2015, infusion of silicon carbide nano-particles in molten magnesium was mentioned as a way to produce a new strong and plastic alloy suitable for use in aeronautics, aerospace, automobile and micro-electronics.[39]
Automobile parts
Silicon-infiltrated carbon-carbon composite is used for high performance "ceramic" brake discs, as it is able to withstand extreme temperatures. The silicon reacts with the graphite in the carbon-carbon composite to become carbon-fiber-reinforced silicon carbide (C/SiC). These discs are used on some road-going sports cars, supercars, as well as other performance cars including the Porsche Carrera GT, the Bugatti Veyron, the Chevrolet Corvette ZR1, Bentleys, Ferraris, Lamborghinis, some specific high performance Audis, and the McLaren P1.[40] Silicon carbide is also used in a sintered form for diesel particulate filters.[41] SiC is also used as an oil additive to reduce friction, emissions, and harmonics.[42][43]
Foundry crucibles
SiC is used in crucibles for holding melting metal in small and large foundry applications.[44][45]
Electric systems
The earliest electrical application of SiC was in lightning arresters in electric power systems. These devices must exhibit high resistance until the voltage across them reaches a certain threshold VT at which point their resistance must drop to a lower level and maintain this level until the applied voltage drops below VT.[46]
It was recognized early on that SiC had such a voltage-dependent resistance, and so columns of SiC pellets were connected between high-voltage power lines and the earth. When a lightning strike to the line raises the line voltage sufficiently, the SiC column will conduct, allowing strike current to pass harmlessly to the earth instead of along the power line. Such SiC columns proved to conduct significantly at normal power-line operating voltages and thus had to be placed in series with a spark gap. This spark gap is ionized and rendered conductive when lightning raises the voltage of the power line conductor, thus effectively connecting the SiC column between the power conductor and the earth. Spark gaps used in lightning arresters are unreliable, either failing to strike an arc when needed or failing to turn off afterwards, in the latter case due to material failure or contamination by dust or salt. Usage of SiC columns was originally intended to eliminate the need for the spark gap in a lightning arrester. Gapped SiC lightning arresters were used as lightning-protection tool and sold under GE and Westinghouse brand names, among others. The gapped SiC arrester has been largely displaced by no-gap varistors that use columns of zinc oxide pellets.[47]
Electronic circuit elements

Power electronic devices
Silicon carbide is a semiconductor in research and early mass-production providing advantages for fast, high-temperature and/or high-voltage devices. The first devices available were Schottky diodes, followed by junction-gate FETs and MOSFETs for high-power switching. Bipolar transistors and thyristors are currently developed.[31] A major problem for SiC commercialization has been the elimination of defects: edge dislocations, screw dislocations (both hollow and closed core), triangular defects and basal plane dislocations.[48] As a result, devices made of SiC crystals initially displayed poor reverse blocking performance though researchers have been tentatively finding solutions to improve the breakdown performance.[49] Apart from crystal quality, problems with the interface of SiC with silicon dioxide have hampered the development of SiC-based power MOSFETs and insulated-gate bipolar transistors. Although the mechanism is still unclear, nitridation has dramatically reduced the defects causing the interface problems.[50] In 2008, the first commercial JFETs rated at 1200 V were introduced to the market,[51] followed in 2011 by the first commercial MOSFETs rated at 1200 V. Beside SiC switches and SiC Schottky diodes (also Schottky barrier diode, SBD) in the popular TO-247 and TO-220 packages, companies started even earlier to implement the bare chips into their power electronic modules. SiC SBD diodes found wide market spread being used in PFC circuits and IGBT power modules.[52] Conferences such as the International Conference on Integrated Power Electronics Systems (CIPS) report regularly about the technological progress of SiC power devices. Major challenges for fully unleashing the capabilities of SiC power devices are:
- Gate drive: SiC devices often require gate drive voltage levels that are different from their silicon counterparts and may be even unsymmetric, for example, +20 V and −5 V.[53]
- Packaging: SiC chips may have a higher power density than silicon power devices and are able to handle higher temperatures exceeding the silicon limit of 150 °C. New die attach technologies such as sintering are required to efficiently get the heat out of the devices and ensure a reliable interconnection.[54]
LEDs
The phenomenon of electroluminescence was discovered in 1907 using silicon carbide and the first commercial LEDs were again based on SiC. Yellow LEDs made from 3C-SiC were manufactured in the Soviet Union in the 1970s[55] and blue ones (6H-SiC) worldwide in the 1980s.[56] The production was soon stopped because gallium nitride showed 10–100 times brighter emission. This difference in efficiency is due to the unfavorable indirect bandgap of SiC, whereas GaN has a direct bandgap which favors light emission. However, SiC is still one of the important LED components – it is a popular substrate for growing GaN devices, and it also serves as a heat spreader in high-power LEDs.[56]
Astronomy
The low thermal expansion coefficient, high hardness, rigidity and thermal conductivity make silicon carbide a desirable mirror material for astronomical telescopes. The growth technology (chemical vapor deposition) has been scaled up to produce disks of polycrystalline silicon carbide up to 3.5 m (11 ft) in diameter, and several telescopes like the Herschel Space Telescope are already equipped with SiC optics,[57][58] as well the Gaia space observatory spacecraft subsystems are mounted on a rigid silicon carbide frame, which provides a stable structure that will not expand or contract due to heat.
Thin filament pyrometry

Silicon carbide fibers are used to measure gas temperatures in an optical technique called thin filament pyrometry. It involves the placement of a thin filament in a hot gas stream. Radiative emissions from the filament can be correlated with filament temperature. Filaments are SiC fibers with a diameter of 15 micrometers, about one fifth that of a human hair. Because the fibers are so thin, they do little to disturb the flame and their temperature remains close to that of the local gas. Temperatures of about 800–2500 K can be measured.[59][60]
Heating elements
References to silicon carbide heating elements exist from the early 20th century when they were produced by Acheson's Carborundum Co. in the U.S. and EKL in Berlin. Silicon carbide offered increased operating temperatures compared with metallic heaters. Silicon carbide elements are used today in the melting of glass and non-ferrous metal, heat treatment of metals, float glass production, production of ceramics and electronics components, igniters in pilot lights for gas heaters, etc.[61]
Nuclear fuel particles
Silicon carbide is an important material in TRISO-coated fuel particles, the type of nuclear fuel found in high temperature gas cooled reactors such as the Pebble Bed Reactor. A layer of silicon carbide gives coated fuel particles structural support and is the main diffusion barrier to the release of fission products.[62]
Nuclear fuel cladding
Silicon carbide composite material has been investigated for use as a replacement for Zircaloy cladding in light water reactors. The composite consists of SiC fibers wrapped around a SiC inner layer and surrounded by an SiC outer layer.[63] Problems have been reported with the ability to join the pieces of the SiC composite.[64]
Jewelry

As a gemstone used in jewelry, silicon carbide is called "synthetic moissanite" or just "moissanite" after the mineral name. Moissanite is similar to diamond in several important respects: it is transparent and hard (9–9.5 on the Mohs scale, compared to 10 for diamond), with a refractive index between 2.65 and 2.69 (compared to 2.42 for diamond). Moissanite is somewhat harder than common cubic zirconia. Unlike diamond, moissanite can be strongly birefringent. For this reason, moissanite jewels are cut along the optic axis of the crystal to minimize birefringent effects. It is lighter (density 3.21 g/cm3 vs. 3.53 g/cm3), and much more resistant to heat than diamond. This results in a stone of higher luster, sharper facets and good resilience. Loose moissanite stones may be placed directly into wax ring moulds for lost-wax casting, as can diamond,[65] as moissanite remains undamaged by temperatures up to 1800 °C. Moissanite has become popular as a diamond substitute, and may be misidentified as diamond, since its thermal conductivity is closer to diamond than any other substitute. Many thermal diamond-testing devices cannot distinguish moissanite from diamond, but the gem is distinct in its birefringence and a very slight green or yellow fluorescence under ultraviolet light. Some moissanite stones also have curved, string-like inclusions, which diamonds never have.[66]
Steel production
Silicon carbide, dissolved in a basic oxygen furnace used for making steel, acts as a fuel. The additional energy liberated allows the furnace to process more scrap with the same charge of hot metal. It can also be used to raise tap temperatures and adjust the carbon and silicon content. Silicon carbide is cheaper than a combination of ferrosilicon and carbon, produces cleaner steel and lower emissions due to low levels of trace elements, has a low gas content, and does not lower the temperature of steel.[67]
Catalyst support
The natural resistance to oxidation exhibited by silicon carbide, as well as the discovery of new ways to synthesize the cubic β-SiC form, with its larger surface area, has led to significant interest in its use as a heterogeneous catalyst support. This form has already been employed as a catalyst support for the oxidation of hydrocarbons, such as n-butane, to maleic anhydride.[68][69]
Carborundum printmaking
Silicon carbide is used in carborundum printmaking – a collagraph printmaking technique. Carborundum grit is applied in a paste to the surface of an aluminium plate. When the paste is dry, ink is applied and trapped in its granular surface, then wiped from the bare areas of the plate. The ink plate is then printed onto paper in a rolling-bed press used for intaglio printmaking. The result is a print of painted marks embossed into the paper.[70]
Graphene production
Silicon carbide is used to produce epitaxial graphene by graphitization at high temperatures. This is considered as one of the promising methods to synthesize graphene at large scale for practical applications.[71][72]
See also
References
- 1 2 3 4 "NIOSH Pocket Guide to Chemical Hazards #0555". National Institute for Occupational Safety and Health (NIOSH).
- ↑ Patnaik, P. (2002). Handbook of Inorganic Chemicals. McGraw-Hill. ISBN 0-07-049439-8.
- 1 2 3 "Properties of Silicon Carbide (SiC)". Ioffe Institute. Retrieved 2009-06-06.
- ↑ Weimer, A. W. (1997). Carbide, nitride, and boride materials synthesis and processing. Springer. p. 115. ISBN 0-412-54060-6.
- ↑ Encyclopædia Britannica, eb.com
- ↑ Acheson, G. (1893) U.S. Patent 492,767 "Production of artificial crystalline carbonaceous material"
- ↑ "The Manufacture of Carborundum- a New Industry". Scientific American. April 7, 1894. Retrieved 2009-06-06.
- ↑ Mabery, Charles F. (1900). "Notes, On Carborundum". Journal of the American Chemical Society. Johnson Reprint Company, via Google Books scan of Harvard University copy. XXII (Part II): 706–707. doi:10.1021/ja02048a014. Retrieved 2007-10-28.
- ↑ Dunwoody, Henry H.C. (1906) U.S. Patent 837,616 Wireless telegraph system (silicon carbide detector)
- ↑ Hart, Jeffrey A.; Stefanie Ann Lenway; Thomas Murtha. "A History of Electroluminescent Displays".
- ↑ Moissan, Henri (1904). "Nouvelles recherches sur la météorité de Cañon Diablo". Comptes rendus. 139: 773–86.
- ↑ Di Pierro S.; Gnos E.; Grobety B.H.; Armbruster T.; Bernasconi S.M. & Ulmer P. (2003). "Rock-forming moissanite (natural α-silicon carbide)". American Mineralogist. 88: 1817–21.
- ↑ Alexander, C. M. O'D. (1990). "In situ measurement of interstellar silicon carbide in two CM chondrite meteorites". Nature. 348 (6303): 715–17. Bibcode:1990Natur.348..715A. doi:10.1038/348715a0.
- ↑ Kelly, Jim. "The Astrophysical Nature of Silicon Carbide". University College London. Retrieved 2009-06-06.
- ↑ Vlasov, A.S. et alia (1991). "Obtaining silicon carbide from rice husks". Refractories and Industrial Ceramics. 32 (9–10): 521–523. doi:10.1007/bf01287542.
- ↑ Zhong, Y.; Shaw, Leon L.; Manjarres, Misael & Zawrah, Mahmoud F. (2010). "Synthesis of Silicon Carbide Nanopowder Using Silica Fume". Journal of American Ceramic Society. 93 (10): 3159. doi:10.1111/j.1551-2916.2010.03867.x.
- 1 2 3 Harris, Gary Lynn (1995). Properties of silicon carbide. IET. p. 19; 170–180. ISBN 0-85296-870-1.
- ↑ Lely, Jan Anthony (1955). "Darstellung von Einkristallen von Silicium Carbid und Beherrschung von Art und Menge der eingebauten Verunreinigungen". Berichte der Deutschen Keramischen Gesellschaft. 32: 229–236.
- ↑ Lely SiC Wafers. Nitride-crystals.com. Retrieved on 2013-05-04.
- ↑ Ohtani, N.; et al. (2001). Nippon Steel Technical Report no. 84 : Large high-quality silicon carbide substrates (PDF). Archived from the original (PDF) on 2010-12-17.
- ↑ Byrappa, K.; Ohachi, T. (2003). Crystal growth technology. Springer. pp. 180–200. ISBN 3-540-00367-3.
- ↑ Bakin, Andrey S. (2006). "SiC Homoepitaxy and Heteroepitaxy". In M. Shur; S. Rumyantsev; M. Levinshtein. SiC materials and devices. 1. World Scientific. pp. 43–76. ISBN 981-256-835-2.
- 1 2 Park, Yoon-Soo (1998). SiC materials and devices. Academic Press. pp. 20–60. ISBN 0-12-752160-7.
- ↑ Pitcher, M. W.; Joray, S. J.; Bianconi, P. A. (2004). "Smooth Continuous Films of Stoichiometric Silicon Carbide from Poly(methylsilyne)". Advanced Materials. 16 (8): 706. doi:10.1002/adma.200306467.
- ↑ Bunsell, A. R.; Piant, A. (2006). "A review of the development of three generations of small diameter silicon carbide fibres". Journal of Materials Science. 41 (3): 823. Bibcode:2006JMatS..41..823B. doi:10.1007/s10853-006-6566-z.
- ↑ Laine, Richard M.; Babonneau, Florence (1993). "Preceramic polymer routes to silicon carbide". Chemistry of Materials. 5 (3): 260. doi:10.1021/cm00027a007.
- ↑ Cheung, Rebecca (2006). Silicon carbide microelectromechanical systems for harsh environments. Imperial College Press. p. 3. ISBN 1-86094-624-0.
- ↑ Morkoç, H.; Strite, S.; Gao, G. B.; Lin, M. E.; Sverdlov, B.; Burns, M. (1994). "Large-band-gap SiC, III-V nitride, and II-VI ZnSe-based semiconductor device technologies". Journal of Applied Physics. 76 (3): 1363. Bibcode:1994JAP....76.1363M. doi:10.1063/1.358463.
- 1 2 Muranaka, T.; Kikuchi, Yoshitake; Yoshizawa, Taku; Shirakawa, Naoki; Akimitsu, Jun (2008). "Superconductivity in carrier-doped silicon carbide". Sci. Technol. Adv. Mater. 9 (4): 044204. Bibcode:2008STAdM...9d4204M. doi:10.1088/1468-6996/9/4/044204. PMC 5099635
 .
. - ↑ Silicon Carbide. Thermal properties. Ioffe Institute Semiconductors Database.
- 1 2 Bhatnagar, M.; Baliga, B.J. (March 1993). "Comparison of 6H-SiC, 3C-SiC, and Si for power devices". IEEE Transactions on Electron Devices. 40 (3): 645–655. Bibcode:1993ITED...40..645B. doi:10.1109/16.199372.
- ↑ Kriener, M.; Muranaka, Takahiro; Kato, Junya; Ren, Zhi-An; Akimitsu, Jun; Maeno, Yoshiteru (2008). "Superconductivity in heavily boron-doped silicon carbide". Sci. Technol. Adv. Mater. 9 (4): 044205. arXiv:0810.0056
 . Bibcode:2008STAdM...9d4205K. doi:10.1088/1468-6996/9/4/044205. PMC 5099636
. Bibcode:2008STAdM...9d4205K. doi:10.1088/1468-6996/9/4/044205. PMC 5099636 .
. - ↑ Yanase, Y. & Yorozu, N. (2008). "Superconductivity in compensated and uncompensated semiconductors". Sci. Technol. Adv. Mater. 9 (4): 044201. Bibcode:2008STAdM...9d4201Y. doi:10.1088/1468-6996/9/4/044201. PMC 5099632
 .
. - ↑ Fuster, Marco A. (1997) "Skateboard grip tape", U.S. Patent 5,622,759
- ↑ Bansal, Narottam P. (2005). Handbook of ceramic composites. Springer. p. 312. ISBN 1-4020-8133-2.
- ↑ "Ceramics for turbine engines". unipass.com. Retrieved 2009-06-06.
- ↑ "Dragon Skin – Most Protective Body Armor – Lightweight". Future Firepower. Retrieved 2009-06-06.
- ↑ "Silicon Carbide". Ceramic Arts Daily.
- ↑ UCLA researchers create exceptionally strong and lightweight new metal
- ↑ "Top 10 Fast Cars". topmost10.com. Archived from the original on 2009-03-26. Retrieved 2009-06-06.
- ↑ O'Sullivan, D.; Pomeroy, M.J.; Hampshire, S.; Murtagh, M.J. (2004). "Degradation resistance of silicon carbide diesel particulate filters to diesel fuel ash deposits". MRS proceedings. 19 (10): 2913–2921. Bibcode:2004JMatR..19.2913O. doi:10.1557/JMR.2004.0373.
- ↑ "SiC Lubrication". Cerma.
- ↑ Studt, P. (1987). "Influence of lubricating oil additives on friction of ceramics under conditions of boundary lubrication". Wear. 115: 185. doi:10.1016/0043-1648(87)90208-0.
- ↑ Friedrichs, Peter; Kimoto, Tsunenobu; Ley, Lothar; Pensl, Gerhard (2011). Silicon Carbide: Volume 1: Growth, Defects, and Novel Applications. John Wiley & Sons. pp. 49–. ISBN 978-3-527-62906-0.
- ↑ Brown, John (1999). Foseco Non-Ferrous Foundryman's Handbook. Butterworth-Heinemann. pp. 52–. ISBN 978-0-08-053187-8.
- ↑ Whitaker, Jerry C. (2005). The electronics handbook. CRC Press. p. 1108. ISBN 0-8493-1889-0.
- ↑ Bayliss, Colin R. (1999). Transmission and distribution electrical engineering. Newnes. p. 250. ISBN 0-7506-4059-6.
- ↑ Chen, H.; Raghothamachar, Balaji; Vetter, William; Dudley, Michael; Wang, Y.; Skromme, B. J. (2006). "Effects of defect types on the performance of devices fabricated on a 4H-SiC homoepitaxial layer". Mater. Res. Soc. Symp. Proc. 911: 169. doi:10.1557/PROC-0911-B12-03.
- ↑ Madar, Roland (2004-08-26). "Materials science: Silicon carbide in contention". Nature. 430 (7003): 974–975. Bibcode:2004Natur.430..974M. doi:10.1038/430974a. PMID 15329702.
- ↑ Chen, Z.; Ahyi, A.C.; Zhu, X.; Li, M.; Isaacs-Smith, T.; Williams, J.R.; Feldman, L.C. (2010). "MOS Characteristics of C-Face 4H-SiC". J. Of Elec. Mat. 39 (5): 526–529. Bibcode:2010JEMat..39..526C. doi:10.1007/s11664-010-1096-5.
- ↑ At 1200 V and 45 Milliohms, SemiSouth Introduces the Industry`s Lowest Resistance SiC Power Transistor for Efficient Power Management. Reuters. 2011-05-05
- ↑ Cree Launches Industry’s First Commercial Silicon Carbide Power MOSFET; Destined to Replace Silicon Devices in High-Voltage (≥ 1200-V) Power Electronics. Cree. 2011-01-17
- ↑ Meißer, Michael (2013). Resonant Behaviour of Pulse Generators for the Efficient Drive of Optical Radiation Sources Based on Dielectric Barrier Discharges. KIT Scientific Publishing. p. 94. ISBN 978-3-7315-0083-4.
- ↑ Horio, Masafumi; Iizuka, Yuji; Ikeda, Yoshinari (2012). "Packaging Technologies for SiC Power Modules" (PDF). Fuji Electric Review. 58 (2): 75–78.
- ↑ Klipstein, Don. "Yellow SiC LED". Retrieved 2009-06-06.
- 1 2 Stringfellow; Gerald B. (1997). High brightness light emitting diodes. Academic Press. pp. 48, 57, 425. ISBN 0-12-752156-9.
- ↑ "The largest telescope mirror ever put into space". European Space Agency. Retrieved 2009-06-06.
- ↑ Petrovsky, Gury T.; Tolstoy, Michael N.; Lubarsky, Sergey V.; Khimitch, Yuri P.; Robb, Paul N.; Tolstoy; Lubarsky; Khimitch; Robb (1994). Stepp, Larry M, ed. "2.7-meter-diameter silicon carbide primary mirror for the SOFIA telescope". Proc. SPIE. Advanced Technology Optical Telescopes V. 2199: 263. Bibcode:1994SPIE.2199..263P. doi:10.1117/12.176195.
- ↑ "Thin-Filament Pyrometry Developed for Measuring Temperatures in Flames". NASA. Retrieved 2009-06-06.
- ↑ Maun, Jignesh D.; Sunderland, PB; Urban, DL (2007). "Thin-filament pyrometry with a digital still camera". Applied Optics. 46 (4): 483–8. Bibcode:2007ApOpt..46..483M. doi:10.1364/AO.46.000483. PMID 17230239.
- ↑ Deshmukh, Yeshvant V. (2005). Industrial heating: principles, techniques, materials, applications, and design. CRC Press. pp. 383–393. ISBN 0-8493-3405-5.
- ↑ López-Honorato, E.; Tan, J.; Meadows, P.J.; Marsh, G.; Xiao, P. (2009). "TRISO coated fuel particles with enhanced SiC properties". Journal of Nuclear Materials. 392 (2): 219. Bibcode:2009JNuM..392..219L. doi:10.1016/j.jnucmat.2009.03.013.
- ↑ Carpenter, David; Ahn, K.; Kao, S.P.; Hejzlar, Pavel; Kazimi, Mujid S. "Assessment of Silicon Carbide Cladding for High Performance Light Water Reactors". Nuclear Fuel Cycle Program, Volume MIT-NFC-TR-098 (2007). Archived from the original on 2012-04-25. Retrieved 2011-10-13.
- ↑ Ames, Nate (June 17, 2010). "SiC Fuel Cladding". Nuclear Fabrication Consortium, nuclearfabrication.org. Archived from the original on April 25, 2012. Retrieved 2011-10-13.
- ↑ Teague, Tyler. Casting Metal Directly onto Stones, Jett Industries
- ↑ O'Donoghue, M. (2006). Gems. Elsevier. p. 89. ISBN 0-7506-5856-8.
- ↑ "Silicon carbide (steel industry)". Retrieved 2009-06-06.
- ↑ Rase, Howard F. (2000). Handbook of commercial catalysts: heterogeneous catalysts. CRC Press. p. 258. ISBN 0-8493-9417-1.
- ↑ Singh, S. K.; Parida, K. M.; Mohanty, B. C.; Rao, S. B. (1995). "High surface area silicon carbide from rice husk: A support material for catalysts". Reaction Kinetics and Catalysis Letters. 54 (1): 29. doi:10.1007/BF02071177.
- ↑ "Printmaking". Bircham Gallery, birchamgallery.co.uk. Retrieved 2009-07-31.
- ↑ de Heer; Walt A. (2010). "Epitaxial graphene". In Sattler, Klaus D. Handbook of Nanophysics (free download). Taylor and Francis. ISBN 1-4200-7538-1. Retrieved 2009-07-31.
- ↑ de Heer, Walt A.; Berger, Claire; Wu, Xiaosong; First, Phillip N.; Conrad, Edward H.; Li, Xuebin; Li, Tianbo; Sprinkle, Michael; Hass, Joanna; Sadowski, Marcin L.; Potemski, Marek; Martinez, Gérard (2007). "Epitaxial graphene" (PDF). Solid State Communications. 143 (1–2): 92. Bibcode:2007SSCom.143...92D. doi:10.1016/j.ssc.2007.04.023. Archived from the original (free download) on 2008-12-09. Retrieved 2009-07-31.
External links
| Wikimedia Commons has media related to Silicon carbide. |
| Wikisource has the text of the 1911 Encyclopædia Britannica article Carborundum. |
- A Brief History of Silicon Carbide Dr J F Kelly, University of London
- Material Safety Data Sheet for Silicon Carbide
- Moissanite on Mindat.org
- CDC – NIOSH Pocket Guide to Chemical Hazards

