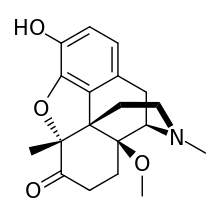
14-Methoxymetopon
 chemical structure | |
| Identifiers | |
|---|---|
| |
| Synonyms | 14-Methoxymetopon |
| CAS Number |
131575-03-6 |
| PubChem (CID) | 5486940 |
| ChemSpider |
4589140 |
| ChEMBL |
CHEMBL326684 |
| Chemical and physical data | |
| Formula | C19H23NO4 |
| Molar mass | 329.39 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
| | |
14-Methoxymetopon is an opiate analogue developed by a team led by Professor Helmut Schmidhammer at the University of Insbruck in the mid 1990s.[1] It is a derivative of metopon in which a methoxy group has been inserted at the 14-position. It is a highly potent analgesic drug that is around 500x stronger than morphine when administered systemically; however, when given spinally or supraspinally, it exhibits analgesic activity up to a million fold greater than morphine.[2] It binds strongly to the μ-opioid receptor and activates it to a greater extent than most similar opioid drugs.[3] This produces an unusual pharmacological profile, and although 14-methoxymetopon acts as a potent μ-opioid full agonist in regard to some effects such as analgesia, a ceiling effect is seen on other effects such as constipation and respiratory depression which is believed to involve interaction with the κ-opioid receptor[4]
See also
References
- ↑ US Patent 5886001 - Agonist compounds
- ↑ King, MA; Su, W; Nielan, CL; Chang, AH; Schütz, J; Schmidhammer, H; Pasternak, GW (2003). "14-Methoxymetopon, a very potent mu-opioid receptor-selective analgesic with an unusual pharmacological profile". European Journal of Pharmacology. 459 (2–3): 203–9. doi:10.1016/S0014-2999(02)02821-2. PMID 12524147.
- ↑ Mahurter, L; Garceau, C; Marino, J; Schmidhammer, H; Tóth, G; Pasternak, GW (2006). "Separation of binding affinity and intrinsic activity of the potent mu-opioid 14-methoxymetopon". The Journal of Pharmacology and Experimental Therapeutics. 319 (1): 247–53. doi:10.1124/jpet.106.105395. PMID 16801454.
- ↑ Freye, E; Schmidhammer, H; Latasch, L (2000). "14-methoxymetopon, a potent opioid, induces no respiratory depression, less sedation, and less bradycardia than sufentanil in the dog". Anesthesia and Analgesia. 90 (6): 1359–64. doi:10.1097/00000539-200006000-00018. PMID 10825321.